Akbar Khan, Doug Andrews, Anneke C Blackburn
Akbar Khan, Douglas Andrews, Medicor Cancer Centres Inc., Toronto, ON M2N 6N4, Canada
Anneke C Blackburn, The John Curtin School of Medical Research, The Australian National University, Canberra, ACT 2601, Australia
Author contributions: Khan A treated the patient and wrote most of the case report; Andrews D treated the patient, designed the natural therapy protocols, and co-wrote the case report; Blackburn AC performed in vitro and in vivo work demonstrating DCA’s effects as a cytostatic agent, and wrote the parts of the case report dealing with the in vitro and in vivo DCA research.
Institutional review board statement: Not applicable.
Informed consent statement: The patient described in this manuscript has given consent to publish her case anonymously.
Conflict-of-interest statement: One of the authors (Khan) administers dichloroacetate therapy for cancer patients through Medicor Cancer Centres at a cost, and without profit. The clinic is owned by a family member of this author. The other authors have nothing to disclose.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Correspondence to: Akbar Khan, MD, Medical Director,
Medicor Cancer Centres Inc., 4576 Yonge St., Suite 301, Toronto,
ON M2N 6N4, Canada. [email protected]
Telephone: +1-416-2270037
Fax: +1-416-2271915
Received: April 30, 2016
Peer-review started: May 3, 2016
First decision: June 17, 2016
Revised: July 23, 2016
Accepted: August 6, 2016
Article in press: August 8, 2016
Published online: October 16, 2016
Abstract
Oral dichloroacetate sodium (DCA) has been investi-gated as a novel metabolic therapy for various cancers since 2007, based on data from Bonnet et al that DCA can trigger apoptosis of human lung, breast and brain cancer cells. Response to therapy in human studies is measured by standard response evaluation criteria for solid tumours definitions, which define “response” by the degree of tumour reduction, or tumour disappearance on imaging.
However, Blackburn et al have demonstrated that DCA can also act as a cytostatic agent in vitro and in vivo , without causing apoptosis (programmed cell death). A case is presented in which oral DCA therapy resulted in tum our stabilization of stage 4 colon cancer in a 57 years old female for a period of nearly 4 years, with no serious toxicity. Since the natural history of stage 4 colon cancer consists of steady progression leading to disability and death, this case highlights a novel use of DCA as a cytostatic agent with a potential to maintain long-term stability of advanced-stage cancer.
Key words: Dichloroacetate; Cancer; Colon; Colorectal; Cytostatic; Stabilization; Growth inhibition; Intravenous
© The Author(s) 2016. Published by Baishideng Publishing Group Inc. All rights reserved.
Core tip: Oral dichloroacetate sodium (DCA) has been investigated as a novel metabolic therapy for various cancers. Response to therapy in human studies is measured by standard response evaluation criteria for solid tumours definitions, which define “response” by the degree of tumour reduction, or tumour disappearance on imaging.
However, DCA can also act as a cytostatic agent, without causing apoptosis (programmed cell death). A case is presented in which oral DCA therapy resulted in tumour stabilization of stage 4 colon cancer in a 57 years old female for a period of nearly 4 years, with no serious toxicity.
Khan A, Andrews D, Blackburn AC. Long-term stabilization of stage 4 colon cancer using sodium dichloroacetate therapy. World J Clin Cases 2016; 4(10): 336-343
Available from: URL: http://www.wjgnet.com/2307-8960/full/v4/i10/336.htm
DOI: http://dx.doi.org/10.12998/wjcc.v4.i10.336
INTRODUCTION
The drug sodium dichloroacetate (DCA) has been investigated as a novel metabolic therapy for various cancers since 2007 when Bonnet et al[1] published a combined in vitro/in vivo rat study demonstrating the efficacy of DCA in treating human lung, breast and brain cancers by inhibition of mitochondrial pyruvate dehydrogenase kinase. Stacpoole et al[2-4] had previously published multiple studies involving DCA for the treatment of congenital lactic acidosis, which is composed of a collection of inherited mitochondrial diseases[5].
These studies established the safety profile of oral DCA in humans. DCA was discovered to be a safe drug with no cardiac, pulmonary, renal or bone marrow toxicity[4]. The most serious common side effect consists of peripheral neuropathy, which is reversible[6]. Delirium has been reported, and is reversible after discontinuation of DCA[7]. Asymptomatic and reversible liver enzyme elevation has been reported in a small percentage of patients[3].
The prior work in congenital lactic acidosis has enabled the rapid progression of DCA into the cancer clinic. Four reports have now been published of cancer clinical trials using DCA, indicating a growing recognition of the potential usefulness of DCA[8-11]. However, these trials treating late stage patients, have only been able to report on treatment for relatively short time periods.
In the initial 2007 paper by Bonnet et al[1], it was reported that DCA reduced mitochondrial membrane potential resulting in selective apoptosis in cancer cells. The mechanism identified was inhibition of aerobic glycolysis (the Warburg effect) and activation of mitochondrial potassium ion channels[1].
Further investigation of DCA confirmed anti-cancer activity in several cancer types including colon[12], prostate[13], ovarian[14], neuroblastoma[15], lung carcinoid[16], cervical[17], endometrial[18], cholangiocarcinoma[19], sarcoma[20] and T-cell lymphoma[21].
Other antineoplastic actions of DCA have also been suggested. These include angiogenesis blockade[22], changes in expression of HIF1-α[23], alteration of pH regulators V-ATPase and MCT1, and other cell survival regulators such as PUMA, GLUT1, Bcl2 and p53[24].
However, in the quest for cytotoxic activity, many in vitro reports use concentrations of DCA that are unlikely to be achieved clinically[25]. Some studies have used restricted concentrations and found DCA to be cytostatic rather than cytotoxic, but able to enhance apoptosis with other agents[26-28].
In the report of successful in vivo DCA treatment of breast cancer, Sun et al[26] found DCA to be cytostatic, inhibiting proliferation without increasing apoptosis. DCA was able to significantly reduce metastatic burden in the lungs of rats in a highly metastatic in vivo model of breast cancer. This suggests a new role for DCA as a cancer stabilizing agent, similar to an anti-angiogenic therapy.
However, to the authors’ knowledge, no human data has yet been published supporting the use of DCA for long-term maintenance of stable disease. As a result of Bonnet’s groundbreaking DCA publication, in early 2007, Khan commenced the clinical use of DCA to treat cancer patients with a poor prognosis or who failed to respond to approved cancer therapies.
A protocol of natural medications was developed to address the dose-limiting neurologic toxicity, in collaboration with a naturopathic physician (Andrews). The oral DCA regimen that was developed included three natural medications acetyl L-carnitine[29-31], R-alpha lipoic acid[32-34] and benfotiamine[35-37], for the primary purpose of neuropathy prevention.
Observational data collected from more than 300 cancer patients with advanced disease revealed measurable benefits from DCA therapy in 60%-70% of cases. The neuropathy risk with inclusion of natural neuroprotective agents was roughly 20% with 20-25 mg/kg per day dosing on a 2 wk on/1 wk off cycle. Reversible liver enzyme elevation was noted in approximately 2% in this patient group (clinic observational data published online at www.medicorcancer.com).
A patient case illustrating the cytostatic effects of oral DCA treatment maintained over several years is presented. This patient had a poor prognosis (median survival of 9-12 mo for stage 4 colorectal cancer using aggressive conventional palliative chemotherapy)[38]. The patient was treated by Khan in cooperation with the naturopathic physician Andrews, who developed a protocol comprised of natural neuroprotective agents.
CASE REPORT
A 57 years old female attended the author’s clinic (Khan) in March 2012 seeking therapy for metastatic colorectal cancer. The patient was originally diagnosed with rectal cancer in mid-2010 when she consulted her doctor for new constipation and low back pain. Colonoscopy was attempted, but the colonoscope could not be advanced due to the presence of a partially-obstructing rectal tumour. Biopsy confirmed moderately differentiated colorectal adenocarcinoma.
Computerized tomography (CT) scan at the time demonstrated stage 4 disease with multiple liver metastases up to 3 cm in diameter, possible tiny lung metastases and an annular rectal carcinoma that was not easily measured (margins of the cancer were difficult to distinguish from the surrounding tissues on CT scan).
The patient underwent a loop ileostomy procedure to bypass the obstruction, and the rectal tumour was not excised. Surgery was followed by chemotherapy consisting of 5-fluorouracil, irinotecan, leucovorin and bevacizumab (FOLFIRI + bevacizumab). Initially the patient responded to chemotherapy with a reduction in liver metastases, a reduction of the primary rectal lesion, and a reduction of blood carcinoembryonic antigen (CEA) marker from 260.9 ng/mL before chemo to 3.5 ng/mL just prior to DCA therapy initiation. The response to chemo then began to plateau. By the time the patient presented to the author’s clinic, chemotherapy was causing minimal disease reduction, and was essentially just maintaining stability.
The patient was previously healthy, and had a 20 year smoking history. She consumed alcohol on occasion. There was a positive family history of colon cancer and gastric cancer. Medications included ongoing chemotherapy as described, hydrogen peroxide enemas, oral vitamin C, occasional oral vitamin D, time- release hydromorphone 32 mg twice a day, and short-acting hydromorphone 2-4 mg orally as needed for “breakthrough” pain. There were no allergies.
Functional enquiry revealed some mild mouth sores related to the ongoing chemotherapy, mild diarrhea (expected with an ileostomy) and mild intermittent rectal bleeding. There was aching/burning lower back and sacral pain up to 6 out of 10 intensity, and mild right shoulder-tip pain exacerbated by chemotherapy (felt to be referred pain related to liver metastases).
Since the chemotherapy was still effective, and the patient was not experiencing any serious side effects, the initial approach was to support the patient’s existing therapy, not replace it. An integrative plan was made in cooperation with a naturopathic physician (Andrews).
The plan consisted of addition of high dose oral vitamin D at 10 000 international units per day, a change of oral vitamin C to vitamin C 50 g intravenous (i.v.) weekly, and addition of dichloroacetate sodium (DCA) 3000 mg i.v. (49 mg/kg) weekly (manufacturer: Tokyo Chemical Industry, United States). To reduce the risk of DCA side effects, 3 natural supplements were prescribed: Alpha lipoic acid (racemic) 500 mg i.v. with each DCA dose, oral R-alpha lipoic acid 150 mg 3 times a day, oral acetyl L-carnitine 500 mg 3 times a day, and oral benfotiamine 80 mg twice a day.
Infusions were planned around chemotherapy infusions (separated by at least 2 d from chemotherapy) to avoid any potential interference or drug interactions. Lipoic acid was not given on chemotherapy days, or within 1 d before or after chemotherapy, since it is a powerful antioxidant and has the potential to reduce chemotherapy efficacy. Integrative therapy began in March 2012. No side effects were noted, so DCA was increased to 4000 mg i.v. (66 mg/kg) weekly. The only side effect noted at the higher DCA dose was mild post-infusion sedation.
Table 1 Blood panel prior to dichloroacetate sodium therapy
| Blood test | Value | Units | Normal range |
|---|---|---|---|
| Hemoglobin | 131 | g/L | 115-155 |
| White cell count | 6.5 | × 109/L | 4.0-11.0 |
| Platelets | 202 | × 109/L | 145-400 |
| Glucose | 5.9 | mmol/L | 2.6-7.0 |
| Urea | 6.5 | mmol/L | 2.5-8.1 |
| Creatinine | 64 | μmol/L | 50-100 |
| Calcium | 2.38 | mmol/L | 2.20-2.65 |
| Albumin | 43 | g/L | 35-52 |
| Bilirubin | 15 | μmol/L | < 23 |
| Sodium | 140 | mmol/L | 136-146 |
| Potassium | 4.2 | mmol/L | 3.7-5.4 |
| Chloride | 102 | mmol/L | 95-108 |
| Alkaline phosphatase | 1861 | U/L | 35-122 |
| LDH | 167 | U/L | 110-215 |
| GGT | 3641 | U/L | < 36 |
| AST | 331 | U/L | < 31 |
| ALT | 31 | U/L | < 36 |
Oral metformin was added to help sensitize the cancer to the chemotherapy, starting at 500 mg orally once a day with titration up to 500 mg 3 times a day[39]. Pregabalin was added to help control the neuropathic sacral pain (started 50 mg daily, titrated up to 50 mg 3 times a day). Chemotherapy side effects included nausea and vomiting (prior to initiation of metformin), and metformin was skipped on days when the patient felt unwell to prevent potential toxicity, should the patient become dehydrated.
Routine baseline blood tests were obtained including complete cell counts, standard metabolic panel, liver enzymes and bilirubin (Table 1). A baseline CT scan was available, which had been performed 2 mo prior to initiation of integrative therapy with DCA.
After 4 mo of integrative therapy as described, a new CT scan was performed (Figure 1), which was reported as “stable and unchanged”, but no measurements were given. An incidental finding of a gallstone was noted (also stable from prior scan). The patient became frustrated that no improvement was noted, and no detailed measurements were indicated in the CT report. An attempt was made to obtain a positron emission tomography scan to clarify live vs necrotic tumours, but government funding could not be obtained and the patient declined to pay privately for the scan.
After some discussion, the patient elected to continue therapy, and obtain future CT scans at a different hospital. By September 2012, increasing chemotherapy side effects including fatigue, nausea and vomiting were noted. A new CT scan revealed that all the liver lesions were “either smaller or no longer identified”. The greatest tumour reduction was only 2 mm however (2.5 cm marker lesion in liver segment 4a reduced to 2.3 cm). There were no new lesions identified.
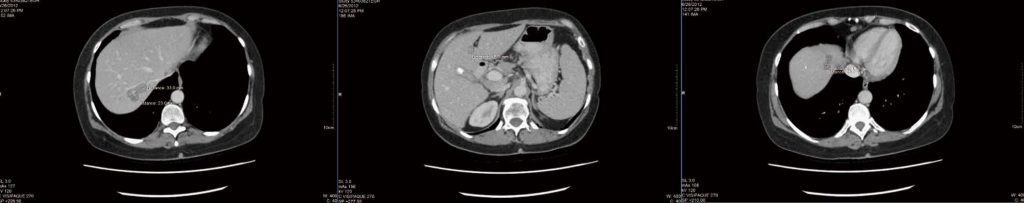
metastasis.
Table 2 Blood panel during dichloroacetate sodium therapy, January 2013
| Blood test | Value | Units | Normal range |
|---|---|---|---|
| Hemoglobin | 134 | g/L | 115-155 |
| White cell count | 5.1 | × 109/L | 4.0-11.0 |
| Platelets | 1421 | × 109/L | 145-400 |
| Glucose | 5.5 | mmol/L | 2.6-7.0 |
| Urea | 4.1 | mmol/L | 2.5-8.1 |
| Creatinine | 57 | μmol/L | 50-100 |
| Calcium | 2.24 | mmol/L | 2.20-2.65 |
| Albumin | 39 | g/L | 35-52 |
| Bilirubin | 11 | μmol/L | < 23 |
| Sodium | 140 | mmol/L | 136-146 |
| Potassium | 4.2 | mmol/L | 3.7-5.4 |
| Chloride | 106 | mmol/L | 95-108 |
| Alkaline phosphatase | 2671 | U/L | 35-122 |
| LDH | 183 | U/L | 110-215 |
| GGT | 8371 | U/L | < 36 |
| AST | 1041 | U/L | < 31 |
| ALT | 100 | U/L | < 36 |
After review of the CT scan, the patient decided to stop all chemotherapy, as well as bevacizumab and metformin. DCA i.v. was continued, and the dose was increased to 4500 mg i.v. weekly. Nausea and vomiting resolved. Pain remained under control.
A new CT scan was obtained after 3 mo, which demonstrated residual rectal tumour with stricture and proximal fecal loading (unchanged), and “liver metastases, not significantly changed”. The patient reported mild numbness of the fingers and toes. There was a further increase in asymptomatic liver enzyme elevations (Table 2). Both of these were diagnosed as DCA side effects. During therapy up to this point, CEA had shown mild fluctuations, but was considered stable overall (Figure 2).
DCA therapy was interrupted for 3 mo to allow resolution of DCA side effects.
During this time, only natural therapies were given (prescribed by Andrews). Acetyl L-carnitine, benfotiamine and alpha lipoic acid were continued to accelerate recovery of DCA neuropathy. Oral curcumin[40] and honokiol (magnolia tree extract) were added in an attempt to maintain cancer control[41]. During the period when DCA was stopped, CEA increased from 4.1 to 5.1 ng/mL (Figure 2). Mild DCA neuropathy had resolved and liver enzymes began to improve.
By March 2013, due to concern over the cost of infusion therapy, it was decided to begin oral DCA therapy. A new baseline CT scan demonstrated a 1 mm increase in a liver segment 7 marker lesion, and a 1 mm increase in a marker aortocaval lymph node, but was reported as “stable appearance of the colon” and “stable liver metastases”.
Oral DCA was initiated at a dose of 500 mg (8.2 mg/kg) twice a day, and neuroprotective supplements consisting of oral acetyl L-carnitine, benfotiamine and R-alpha lipoic acid were continued. Supplements were given continuously, and DCA was given on a 2 wk on/1 wk off cycle.
In December 2013, the pain medication was transitioned from hydromorphone to methadone 10 mg 3 times a day, for simplicity, improved pain control and cost savings.
The patient continued on this regimen with regular CT scans every 3 to 6 mo. The patient became less compliant with regular blood testing due to a busy work schedule. She remained highly functional (ECOG level 1) with mild chronic DCA neuropathy that was controlled and did not affect her daily function.
An attempt was made to increase DCA to 500 mg 3 times a day, but this resulted in significant asymptomatic liver enzyme elevation and increase in neuropathy. As a result, a DCA dose of 500 mg twice a day was resumed after a brief therapy interruption.
Ongoing CT scans continued to reveal stable disease (Figure 3), with no new lesions appearing. Overall CEA was not significantly changed from the initiation of DCA therapy (CEA of 3.5 at the start of DCA therapy, to CEA of 3.7 after nearly 4 years of therapy). General blood panel was also favourable at the 3 year mark (Table 3) and after the 4 year mark (Table 4).
In summary, after receiving conventional chemotherapy for approximately 18 mo, the patient received intravenous DCA therapy with concurrent chemotherapy for approximately 6 mo, followed by intravenous and oral DCA therapy with no concurrent conventional cancer therapy for nearly 4 years. During the treatment with oral DCA, the patient experienced stable disease by CT scans, and stable disease by CEA tumour marker measurement. She also was clinically stable with no escalation of methadone dose, maintenance of ECOG level 1 function, stable mild DCA neuropathy, and she was able to run her own business successfully.
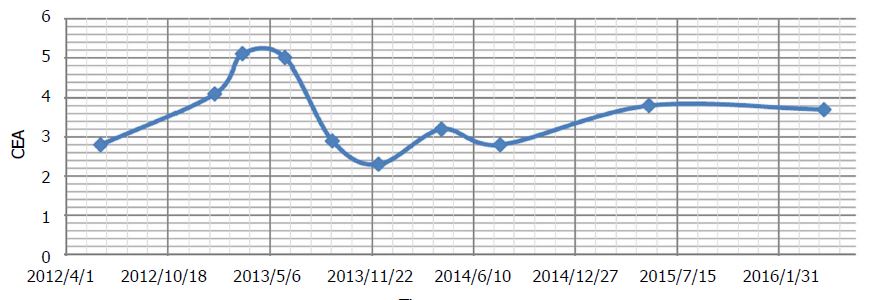
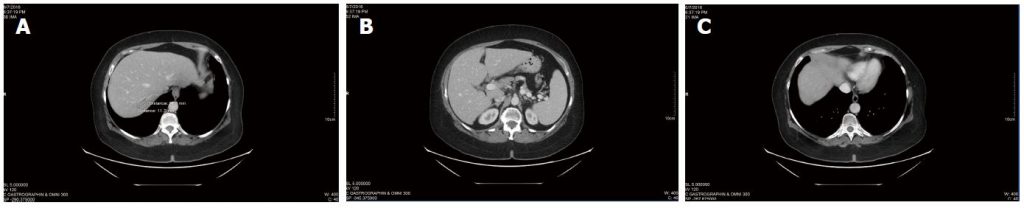
Table 3 Blood panel during dichloroacetate sodium therapy, May 2015
| Blood test | Value | Units | Normal range |
|---|---|---|---|
| Hemoglobin | 134 | g/L | 115-155 |
| White cell count | 7.7 | × 109/L | 4.0-11.0 |
| Platelets | 173 | × 109/L | 145-400 |
| Glucose | 5.3 | mmol/L | 2.6-7.0 |
| Urea | 5.1 | mmol/L | 2.5-8.1 |
| Creatinine | 70 | μmol/L | 50-100 |
| Calcium | 2.37 | mmol/L | 2.20-2.65 |
| Albumin | – | g/L | 35-52 |
| Bilirubin | 8 | μmol/L | < 23 |
| Sodium | 144 | mmol/L | 136-146 |
| Potassium | 4.1 | mmol/L | 3.7-5.4 |
| Chloride | 104 | mmol/L | 95-108 |
| Alkaline phosphatase | – | U/L | 35-122 |
| LDH | 174 | U/L | 110-215 |
| GGT | 1561 | U/L | < 36 |
| AST | 30 | U/L | < 31 |
| ALT | 25 | U/L | < 36 |
Table 4 Blood panel during dichloroacetate sodium therapy, April 2016
| Blood test | Value | Units | Normal range |
|---|---|---|---|
| Hemoglobin | 133 | g/L | 115-155 |
| White cell count | 5.2 | × 109/L | 4.0-11.0 |
| Platelets | 155 | × 109/L | 145-400 |
| Glucose | – | mmol/L | 2.6-7.0 |
| Urea | 4.9 | mmol/L | 2.5-8.1 |
| Creatinine | – | μmol/L | 50-100 |
| Calcium | 2.39 | mmol/L | 2.20-2.65 |
| Albumin | 42 | g/L | 35-52 |
| Bilirubin | 9 | μmol/L | < 23 |
| Sodium | 142 | mmol/L | 136-146 |
| Potassium | 4 | mmol/L | 3.7-5.4 |
| Chloride | 102 | mmol/L | 95-108 |
| Alkaline phosphatase | 101 | U/L | 35-122 |
| LDH | 156 | U/L | 110-215 |
| GGT | 1491 | U/L | < 36 |
| AST | 30 | U/L | < 31 |
| ALT | 28 | U/L | < 36 |
DISCUSSION
This case of DCA therapy in a patient with advanced stage 4 colon cancer demonstrates long-term stable disease according to clinical, biochemical and radiologic criteria.
The duration of stability while on DCA without other active chemotherapy is currently 46 mo (nearly 4 years), with a survival time since the initial diagnosis of stage 4 colorectal cancer of 6 years.
Based on the National Cancer Institute’s SEER cancer statistics review 1975-2011, the 5-year relative survival rate for females diagnosed with stage IV colon/rectal cancer was 14.4% (http://seer.cancer.gov/csr/1975_2013/). While it cannot be definitively concluded that DCA has been efficacious, survival for this length of time in the absence of ongoing chemotherapy would be of relatively low probability.
Cytostatic rather than cytotoxic effects of DCA on colorectal and other cancer cells have been reported and support this clinical finding[23,27,42-44]. To date the patient remains clinically well and she remains on DCA therapy.
In addition to the maintenance of stable disease, this case demonstrates the tolerability of oral DCA in a cancer patient for much longer time periods than those currently reported from the published clinical trials in cancer patients. Chu et al[11] reported on 24 patients treated for a median time of 2 mo at either 6.25 or 12.5 mg/kg BID, on continuous oral DCA without neuroprotective supplements.
They concluded that the recommended phase 2 dose was 6.25 mg/kg BID (12.5 mg/kg per day), with careful monitoring of neuropathy being needed. Dunbar et al[9] recommended 5 mg/kg BID as a starting dose for most patients, with their trial administering 4, 8 or 12.5 mg/kg BID continuously (median time on DCA 34 d), also without neuroprotective supplements.
The patient in this report took 500 mg BID, equivalent to 8.2 mg/kg BID, 2 wk on/1 wk off, but could not tolerate this dose three times a day (total of 25 mg/kg per day). Dunbar et al[9] suggest that genotyping for polymorphisms in GSTZ1, the DCA metabolizing enzyme in the liver which is inactivated with ongoing DCA usage[45], should be considered in determining the starting dose for patients.
However further work is necessary to gather convincing numbers of genotypes and dose-tolerance data. A clinical trial of DCA in multiple myeloma patients is currently underway to contribute to this pool of data (Australia New Zealand Clinical Trials Register #ACTRN12615000226505, http://www.anzctr.org.au).
Further studies are required to determine the optimal dosage regime for maximum tolerable acute or chronic treatment with DCA, and indeed what dose is required for efficacy.
The case presented indicates DCA holds great promise as a cancer therapy. The patient achieved a significant benefit from her therapy, with mild side effects and no hematological, cardiac, pulmonary or renal toxicity. Some hepatic toxicity was observed (Table 2), which was easily managed by DCA therapy interruption followed by dose adjustment.
Mild reversible peripheral neurotoxicity was reported. Natural therapies that were combined with DCA (acetyl L-carnitine, alpha lipoic acid and benfotiamine) assisted the patient with reduction of side effects, but are not known to function as cancer therapies.
As of this writing, no active clinical trials exist investigating the human use of DCA as a cytostatic agent. Due to the fact that DCA is off patent, raising adequate funds to support large scale human trials is a serious challenge. It is hoped that this case exemplifying the benefits of oral DCA will stimulate further clinical investigation.
Based on our clinical experience, combined with existing publications, off-label DCA therapy is an option for patients with limited available conventional treatments, once they understand and accept the risks and benefits of therapy.
This case report shows that even in advanced stage disease, DCA has the potential to prolong life without impacting a patient’s quality of life, as compared to chemotherapy with its frequent debilitating side effects or compromise in physiological function. Given its reasonable cost and modest toxicity, DCA deserves further investigation.
ACKNOWLEDGMENTS
The authors wish to thank Dr. Humaira Khan for her assistance, and also the patient for her support and consent to publish her case.
COMMENTS
Case characteristics
The 57 years old female patient presented with constipation and lower back pain.
Clinical diagnosis
The patient was diagnosed with a partially-obstructing rectal cancer.
Laboratory diagnosis
Elevated carcinoembryonic antigen tumour marker.
Imaging diagnosis
Rectal mass seen on colon colonoscopy.
Pathological diagnosis
Moderately differentiated colorectal adenocarcinoma.
Treatment
Loop ileostomy followed by chemotherapy consisting of 5-fluorouracil, irinotecan, leucovorin and bevacizumab, then addition of dichloroacetate sodium (DCA), then DCA without chemo for nearly 4 years.
Related reports
Computerized tomography scan reports demonstrate reduction of cancer with combined chemotherapy + DCA, then stable disease for nearly 4 years with DCA and no chemo.
Term explanation
DCA: Dichloroacetate sodium; RECIST: Response evaluation criteria for solid tumours; ECOG: Eastern cooperative oncology group; SEER: Surveillance, epidemiology and end results.
Experiences and lessons
DCA is not only a pro-apoptotic drug, but may also act as a cytostatic agent, and can thus achieve long-term stabilization of advanced cancer without serious side effects, as illustrated by this rectal cancer case.
Peer-review
DCA, the sodium salt of dichloroacetate, is a cheap chemical compound that has shown some clear potential as an alternative cancer treatment, which has been used in a number of trials with people suffering from brain cancer, or glioblastoma. This is a well-written case report in which oral DCA therapy resulted in tumor stabilization of stage 4 colon cancer in a 57 years old female for a period of over 3 years, with no serious toxicity. This report covers what it promises to.
The authors do a solid job of explaining the basics of DCA therapy and its role in different tumor types. Along with the addition of mechanisms of action against cancer cells and therapeutic potential of DCA, the authors provide a good resource for readers who are more unfamiliar with DCA therapy but also provide detail.
REFERENCES
1 Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Bonnet S, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 2007; 11: 37-51 [PMID: 17222789 DOI: 10.1016/j.ccr.2006.10.020]2 Stacpoole PW, Kurtz TL, Han Z, Langaee T. Role of dichloroacetate in the treatment of genetic mitochondrial diseases. Adv Drug Deliv Rev 2008; 60: 1478-1487 [PMID: 18647626 DOI: 10.1016/ j.addr.2008.02.014]
3 Stacpoole PW, Gilbert LR, Neiberger RE, Carney PR, Valenstein E, Theriaque DW, Shuster JJ. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics 2008; 121: e1223-e1228 [PMID: 18411236 DOI: 10.1542/peds.2007-2062]
4 Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O’Brien RG, Perkins LA, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics 2006; 117: 1519-1531 [PMID: 16651305 DOI: 10.1542/peds.2005-1226]
5 Berendzen K, Theriaque DW, Shuster J, Stacpoole PW. Therapeutic potential of dichloroacetate for pyruvate dehydrogenase complex deficiency. Mitochondrion 2006; 6: 126-135 [PMID: 16725381 DOI: 10.1016/j.mito.2006.04.001]
6 Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar WS, Hong X, Gooch CL, Mao X, Pascual JM, Hirano M, Stacpoole PW, DiMauro S, De Vivo DC. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology 2006; 66: 324-330 [PMID: 16476929 DOI: 10.1212/01. wnl.0000196641.05913.27]
7 Brandsma D, Dorlo TP, Haanen JH, Beijnen JH, Boogerd W. Severe encephalopathy and polyneuropathy induced by dichloroacetate. J Neurol 2010; 257: 2099-2100 [PMID: 20632025 DOI: 10.1007/ s00415-010-5654-9]
8 Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2010; 2: 31ra34 [PMID: 20463368 DOI: 10.1126/scitranslmed.3000677]
9 Dunbar EM, Coats BS, Shroads AL, Langaee T, Lew A, Forder JR, Shuster JJ, Wagner DA, Stacpoole PW. Phase 1 trial of dichloroacetate (DCA) in adults with recurrent malignant brain tumors. Invest New Drugs 2014; 32: 452-464 [PMID: 24297161 DOI: 10.1007/s10637-013-0047-4]
10 Garon EB, Christofk HR, Hosmer W, Britten CD, Bahng A, Crabtree MJ, Hong CS, Kamranpour N, Pitts S, Kabbinavar F, Patel C, von Euw E, Black A, Michelakis ED, Dubinett SM, Slamon DJ. Dichloroacetate should be considered with platinumbased chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer. J Cancer Res Clin Oncol 2014; 140: 443-452 [PMID: 24442098 DOI: 10.1007/ s00432-014-1583-9]
11 Chu QS, Sangha R, Spratlin J, Vos LJ, Mackey JR, McEwan AJ, Venner P, Michelakis ED. A phase I open-labeled, single-arm, dose-escalation, study of dichloroacetate (DCA) in patients with advanced solid tumors. Invest New Drugs 2015; 33: 603-610 [PMID: 25762000 DOI: 10.1007/s10637-015-0221-y]
12 Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. Br J Cancer 2010; 102: 1746-1752 [PMID: 20485289 DOI: 10.1038/sj.bjc.6605701]
13 Cao W, Yacoub S, Shiverick KT, Namiki K, Sakai Y, Porvasnik S, Urbanek C, Rosser CJ. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. Prostate 2008; 68: 1223-1231 [PMID: 18465755 DOI: 10.1002/pros.20788]
14 Saed GM, Fletcher NM, Jiang ZL, Abu-Soud HM, Diamond MP. Dichloroacetate induces apoptosis of epithelial ovarian cancer cells through a mechanism involving modulation of oxidative stress. Reprod Sci 2011; 18: 1253-1261 [PMID: 21701041 DOI: 10.1177/1 933719111411731]
15 Vella S, Conti M, Tasso R, Cancedda R, Pagano A. Dichloroacetate inhibits neuroblastoma growth by specifically acting against malignant undifferentiated cells. Int J Cancer 2012; 130: 1484-1493 [PMID: 21557214 DOI: 10.1002/ijc.26173]
16 Fiebiger W, Olszewski U, Ulsperger E, Geissler K, Hamilton G. In vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. Clin Transl Oncol 2011; 13: 43-49 [PMID: 21239354 DOI: 10.1007/s12094-011-0615-z]
17 Liu D, Liu S, Jing X, Li X, Li W, Huang Y. Necrosis of cervical carcinoma by dichloroacetate released from electrospun polylactide mats. Biomaterials 2012; 33: 4362-4369 [PMID: 22425553 DOI: 10.1016/j.biomaterials.2012.02.062]
18 Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol 2008; 109: 394-402 [PMID: 18423823 DOI: 10.1016/j.ygyno.2008.01.038]
19 Ishiguro T, Ishiguro R, Ishiguro M, Iwai S. Co-treatment of dichloroacetate, omeprazole and tamoxifen exhibited synergistically antiproliferative effect on malignant tumors: in vivo experiments and a case report. Hepatogastroenterology 2012; 59: 994-996 [PMID: 22580646 DOI: 10.5754/hge10507]
20 Sorokina LV, Pyatchanina TV, Didenko GV, Kaplia AA, Khyzhnyak SV. The influence of sodium dichloroacetate on the oxidative processes in sarcoma 37. Exp Oncol 2011; 33: 216-221 [PMID: 22217710]
21 Kumar A, Kant S, Singh SM. Novel molecular mechanisms of antitumor action of dichloroacetate against T cell lymphoma: Implication of altered glucose metabolism, pH homeostasis and cell survival regulation. Chem Biol Interact 2012; 199: 29-37 [PMID: 22705712 DOI: 10.1016/j.cbi.2012.06.005]
22 Sutendra G, Dromparis P, Kinnaird A, Stenson TH, Haromy A, Parker JM, McMurtry MS, Michelakis ED. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene 2013; 32: 1638-1650 [PMID: 22614004 DOI: 10.1038/onc.2012.198]
23 Shahrzad S, Lacombe K, Adamcic U, Minhas K, Coomber BL. Sodium dichloroacetate (DCA) reduces apoptosis in colorectal tumor hypoxia. Cancer Lett 2010; 297: 75-83 [PMID: 20537792 DOI: 10.1016/j.canlet.2010.04.027]
24 Anderson KM, Jajeh J, Guinan P, Rubenstein M. In vitro effects of dichloroacetate and CO2 on hypoxic HeLa cells. Anticancer Res 2009; 29: 4579-4588 [PMID: 20032407]
25 Kankotia S, Stacpoole PW. Dichloroacetate and cancer: new home for an orphan drug? Biochim Biophys Acta 2014; 1846: 617-629 [PMID: 25157892 DOI: 10.1016/j.bbcan.2014.08.005]
26 Sun RC, Board PG, Blackburn AC. Targeting metabolism with arsenic trioxide and dichloroacetate in breast cancer cells. Mol Cancer 2011; 10: 142 [PMID: 22093145 DOI: 10.1186/1476-4598-10-142]
27 Stockwin LH, Yu SX, Borgel S, Hancock C, Wolfe TL, Phillips LR, Hollingshead MG, Newton DL. Sodium dichloroacetate selectively targets cells with defects in the mitochondrial ETC. Int J Cancer 2010; 127: 2510-2519 [PMID: 20533281 DOI: 10.1002/ijc.25499]
28 Gang BP, Dilda PJ, Hogg PJ, Blackburn AC. Targeting of two aspects of metabolism in breast cancer treatment. Cancer Biol Ther 2014; 15: 1533-1541 [PMID: 25482950 DOI: 10.4161/15384047.2014.955992]
29 De Grandis D. Acetyl-L-carnitine for the treatment of chemotherapy- induced peripheral neuropathy: a short review. CNS Drugs 2007; 21 Suppl 1: 39-43; discussion 45-46 [PMID: 17696592]
30 Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, Crinò L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori 2005; 91: 135-138 [PMID: 15948540]
31 Evans JD, Jacobs TF, Evans EW. Role of acetyl-L-carnitine in the treatment of diabetic peripheral neuropathy. Ann Pharmacother 2008; 42: 1686-1691 [PMID: 18940920 DOI: 10.1345/aph.1L201]
32 Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJ. Alpha lipoic Acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol 2012; 2012: 456279 [PMID: 22331979 DOI: 10.1155/2012/456279]
33 Liu F, Zhang Y, Yang M, Liu B, Shen YD, Jia WP, Xiang KS. Curative effect of alpha-lipoic acid on peripheral neuropathy in type 2 diabetes: a clinical study. Zhonghua Yixue Zazhi 2007; 87: 2706-2709 [PMID: 18167250]
34 Ziegler D, Hanefeld M, Ruhnau KJ, Meissner HP, Lobisch M, Schütte K, Gries FA. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia 1995; 38: 1425-1433 [PMID: 8786016]
35 Winkler G, Kempler P. Pathomechanism of diabetic neuropathy: background of the pathogenesis-oriented therapy. Orv Hetil 2010; 151: 971-981 [PMID: 20519180 DOI: 10.1556/OH.2010.28898]
36 Ang CD, Alviar MJ, Dans AL, Bautista-Velez GG, Villaruz-Sulit MV, Tan JJ, Co HU, Bautista MR, Roxas AA. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev 2008: CD004573 [PMID: 18646107 DOI: 10.1002/14651858.CD004573.pub3]
37 Winkler G, Pál B, Nagybéganyi E, Ory I, Porochnavec M, Kempler P. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittelforschung 1999; 49: 220-224 [PMID: 10219465 DOI: 10.1055/s-0031-1300405]
38 Loupakis F, Cremolini C, Masi G, Lonardi S, Zagonel V, Salvatore L, Cortesi E, Tomasello G, Ronzoni M, Spadi R, Zaniboni A, Tonini G, Buonadonna A, Amoroso D, Chiara S, Carlomagno C, Boni C, Allegrini G, Boni L, Falcone A. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014; 371: 1609-1618 [PMID: 25337750 DOI: 10.1056/ NEJMoa1403108]
39 Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res 2009; 69: 7507-7511 [PMID: 19752085 DOI: 10.1158/0008-5472.can-09-2994]
40 Cao A, Li Q, Yin P, Dong Y, Shi H, Wang L, Ji G, Xie J, Wu D. Curcumin induces apoptosis in human gastric carcinoma AGS cells and colon carcinoma HT-29 cells through mitochondrial dysfunction and endoplasmic reticulum stress. Apoptosis 2013; 18: 1391-1402 [PMID: 23881281 DOI: 10.1007/s10495-013-0871-1]
41 Wynn ML, Consul N, Merajver SD, Schnell S. Inferring the Effects of Honokiol on the Notch Signaling Pathway in SW480 Colon Cancer Cells. Cancer Inform 2014; 13: 1-12 [PMID: 25392689 DOI: 10.4137/CIN.S14060]
42 Delaney LM, Ho N, Morrison J, Farias NR, Mosser DD, Coomber BL. Dichloroacetate affects proliferation but not survival of human colorectal cancer cells. Apoptosis 2015; 20: 63-74 [PMID: 25344893 DOI: 10.1007/s10495-014-1046-4]
43 Sun RC, Fadia M, Dahlstrom JE, Parish CR, Board PG, Blackburn AC. Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat 2010; 120: 253-260 [PMID: 19543830 DOI: 10.1007/s10549-009-0435-9]
44 Sánchez-Aragó M, Chamorro M, Cuezva JM. Selection of cancer cells with repressed mitochondria triggers colon cancer progression. Carcinogenesis 2010; 31: 567-576 [PMID: 20080835 DOI: 10.1093/ carcin/bgq012]
45 Tzeng HF, Blackburn AC, Board PG, Anders MW. Polymorphismand species-dependent inactivation of glutathione transferase zeta by dichloroacetate. Chem Res Toxicol 2000; 13: 231-236 [PMID: 10775321]
Related content: