Wolfgang Fiebiger1, Ulrike Olszewski2, Ernst Ulsperger2, Klaus Geissler2, Gerhard Hamilton2,3
1 Department of Internal Medicine I, Division of Oncology, St. Poelten Hospital, St. Poelten, Austria
2 Ludwig Boltzmann Cluster of Translational Oncology, C/o Balderichgasse, 26/13, 1170 Vienna, Austria
e-mail: [email protected]
G. Hamilton
3 Department of Surgery, Medical University Vienna, Vienna, Austria
Received: 1 April 2010
Accepted: 6 June 2010
Abstract
Introduction: Chemotherapy for advanced well-differentiated carcinoids is characterised by low response rates and short duration of responses. The present study aimed to assess the in vitro activity of novel platinum-based chemotherapeutic drugs in combination with dichloroacetate (DCA), a sensitiser to apoptosis, against lung carcinoid cell lines.
Methods: Three permanent cell lines (UMC-11, H727 and H835) were exposed to 14 different established cytotoxic drugs and the novel platinum-based compounds as satraplatin, JM118 and picoplatin in combination with DCA, and viability of the cells was measured using a tetrazolium based dye assay.
Results: With exception of the highly chemoresistant UMC- 11 line, the carcinoid cell lines (H727, H835) were sensitive to the majority of chemotherapeutics in vitro. Among the platinum-based drugs, carboplatin and oxaliplatin showed highest efficacy. H835 cells growing as multicellular spheroids were 2.7-8.7-fold more resistant to picoplatin, satraplatin and its metabolite compared to single cell suspensions. DCA (10 mM) inhibited the growth of UMC- 11 cells by 22% and sensitised these highly resistant cells to carboplatin, satraplatin and JM118 1.4-2.4-fold.
Conclusion: The highly resistant UMC-11 lung carcinoid cells are sensitive to carboplatin, oxaliplatin and the satraplatin metabolite JM118, but multicellular spheroidal growth, as observed in the H835 cell line and pulmonary tumourlets, seems to increase chemoresistance markedly. The activity of carboplatin and JM118 is significantly and specifically increased in combination with the apoptosis sensitiser DCA that promotes mitochondrial respiration over aerobic glycolysis. In summary, among the novel platinum drugs satraplatin has the potential for treatment of lung carcinoids and DCA potentiates the cytotoxicity of selected platinum drugs.
Keywords: Carcinoid; Chemosensitivity; Drug resistance; Platinum complex; Picoplatin; Satraplatin; Dichloroacetate
INTRODUCTION
Carcinoids derive from enterochromaffin cells of the neuroendocrine cell system that is widely distributed in the body [1–3]. These rather rare tumours have an incidence of 2.0–2.5 cases per 100,000 people for clinically conspicuous carcinoids [4]. Foregut carcinoids comprise 20% of all carcinoid tumours and include thymic and lung (representing 2% of primary lung cancers) in addition to gastric and duodenal carcinoids [5]. According to their histologic characteristics, these heterogenous tumours are classifi ed either as well-differentiated neuroendocrine tumours with small cells and regular nuclei (typical carcinoid) or well differentiated neuroendocrine carcinomas with nuclear atypia and necrotic areas (atypical carcinoid) [6, 7]. The “carcinoid syndrome”, which is induced by secretion of vasoactive substances, occurs in less than 5% of pulmonary carcinoids [8]. From 13 to 22% of patients have distant metastases at time of presentation [4]. Primary diagnostic procedures include biochemical testing, particularly analysis of serotonin and urinary 5-hydroxyindoleacetic acid (5-HIAA). The most sensitive technique for localisation of the tumours is somatostatin receptor scintigraphy supplemented by CT for visualisation of liver metastases and concomitant biopsy for histopathological verification [9]. Since more than 80% of carcinoid tumours express somatostatin receptor type 2, 111indium-DTPA-octreotide as a tracer may be capable of predicting the usefulness of a forthcoming somatostatin analogue therapy [10, 11].
Surgical cure of lung carcinoid patients can be achieved by resection, but a fraction of tumours give rise to widespread metastasis within two to four years after primary operation [12]. In contrast to the more indolent well-differentiated neuroendocrine tumours exhibiting metastases in less than 15% of cases and revealing a five-year survival rate of more than 90%, well-differentiated neuroendocrine carcinomas are more aggressive, with metastases in 30– 50% of cases and a five-year survival rate between 40 and 60% [1, 2, 13]. Patients with malignant carcinoids show a five-year survival rate of about 20% with a median survival of two years in the presence of liver metastases [14–16]. Medical treatment of metastatic disease includes somatostatin analogues, α-interferons and chemotherapy [1, 17].
Clinical trials are usually small with variable inclusion criteria and study design because of the rarity of these tumours [1, 16]. Basically, chemotherapy can be considered for patients with progressive disease under noncytotoxic therapy, provided it is associated with significant symptoms and poor prognosis. Doxorubicin, 5-fluorouracil (5-FU), dacarbazine, streptozotocin, cyclophosphamide, cisplatin and etoposide have been used as single agents and in combinations resulting in response rates of less than 20% only for short periods and therefore have not been recommended for clinical routine practice [1, 3, 16–18].
In the present study, we tested the in vitro activity of newer chemotherapeutic drugs including gemcitabine, camptothecin, oxaliplatin and paclitaxel in comparison to cytotoxic drugs currently used for chemotherapy employing the three permanent lung carcinoid cell lines UMC11, H727 and H835. Lung carcinoids are closely related to small-cell lung cancer (SCLC), which is primarily treated with a combination of cisplatin and etoposide [3,19]. Third-generation platinum-based drugs, such as satraplatin and picoplatin, are currently under investigation for SCLC in clinical trials and maybe also active against pulmonary carcinoids [20]. Therefore, the oral prodrug satraplatin [JM216; bisacetatoammine dichlorido cyclohexylamine platinum(IV)], its active metabolites JM149 [cis-ammine dichlorido (cyclohexylamine) dihydroxidoplatinum(IV)] and JM118 [cis-ammine dichlorido cyclohexylamine platinum(II)], as well as picoplatin [JM473, ZD-0473, AMD-473; cis-ammine dichlorido 2-methylpyridine platinum(II)], were studied for their cytotoxic potency in vitro using the three lung carcinoid cell lines. The efficacy of platinum complexes is expected to be improved further in combination with dichloroacetate (DCA), which targets pyruvate dehydrogenase kinase. Thus, the additive effect of this compound on the cytotoxicity of the platinum drugs was furthermore investigated [21].
Materials and methods
Chemicals and cell lines
Gemcitabine was obtained from Eli Lilly (London, UK), dacarbazine from Medac (Hamburg, Germany), oxaliplatin from Sanofi (Paris, France), and all other drugs were from Sigma-Aldrich (St. Louis, MO, USA). Satraplatin and its metabolites JM149 and JM118 as well as picoplatin were custom-synthesised by Chiracon (Luckenwalde, Germany). Sodium DCA was prepared as 1 M stock solution. National Cancer Institute (NCI) UMC-11, H727 and H835 pulmonary carcinoid cell lines were purchased from the American Tissue Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI-1640 medium supplemented with 10% foetal bovine serum and 4 mM glutamine (Seromed, Berlin, Germany). UMC-11 and H727 cells were subcultured by trypsinisation (2.5% trypsin/EDTA solution, Boehringer Mannheim, Germany), and H835 cells growing in suspension, were maintained by replacement of medium.
Cell cycle distribution and doubling time assays
In order to analyse cell cycle distribution, aliquots of cell cultures were washed with PBS, resuspended in 100 µl PBS and fixed with 1 ml 70% ethanol at –20°C for 30 min. Then, cells were centrifuged, washed with PBS and stained with a solution of 20 µg/ml propidium iodide (PI) in 0.5% Triton X100/PBS supplemented with 5 µg/ml RNAse A for 24 h at room temperature. Histograms were acquired by a FACS Scan flow cytometry system (Becton Dickinson, Mountain View, CA, USA), and cell cycle distribution was calculated using Multicycle AV software (Phoenix, San Diego, CA, USA). Cell line doubling times were determined by daily measurements of cell numbers from three independent cultures for six consecutive days using a microcellcounter (Sysmex, Tokyo, Japan). H835 cell clusters were dispersed by trypsin treatment to obtain single cell suspensions for counting.
Cell proliferation assay
Cells were harvested, counted and distributed to microtiter plates in 100 µl medium at a density of 1×104 cells/well. Appropriate dilutions of test compounds were added to a total volume of 200 µl/well and plates incubated under tissue culture conditions for four days. Stock solutions of the compounds were prepared in either 70% ethanol or dimethyl sulphoxide and diluted more than 100-fold for the assays. Solvent controls were included in all tests. Doseresponse curves were obtained by assessing cell proliferation at twofold drug dilutions in triplicate and used for calculation of IC50 values. Cell growth was quantified using a modified tetrazolium dye assay (MTT; EZ4U, Biomedica, Vienna, Austria) and by measurement of the reduced formazane dye at 450 nm wavelength (medium control set to 100% proliferation).
Statistical analysis
Differences between two independent groups were determined using ANOVA and the Mann–Whitney U-test. p<0.05 was regarded as statistically significant. All calculations were done using Statistica software (Statsoft, Tulsa, OK, USA). Cell line doubling times were analysed using an online calculator (Roth V. 2006, available from ).
Results
Cell cycle distribution and doubling time of the three lung carcinoid cell lines
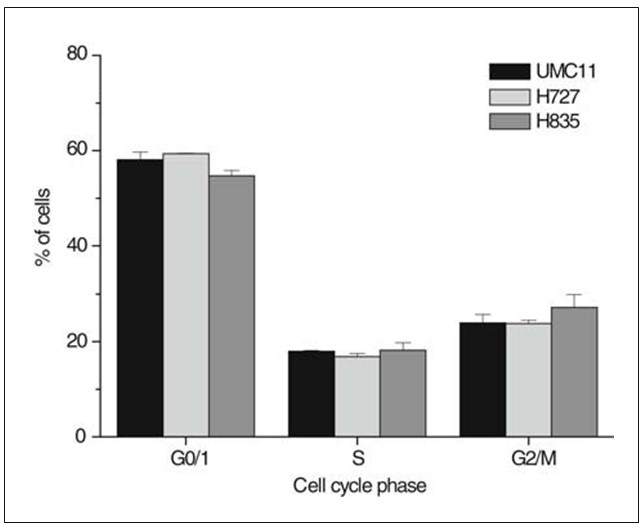
Percentages of carcinoid cells in specific cell cycle phases were determined by PI staining of fixed cells derived from subconfluent cultures, flow cytometric analysis and curve fitting of the resulting histograms (Fig. 1). The cell lines exhibited a fraction of S-phase cells exceeding 17% and G0/1 fractions below 54% of the total cell population, respectively. The S-phase fractions of the UMC-11, H727 and H835 carcinoid cell lines were not significantly different. Cell line doubling times were determined by daily counting of cells from six independent cultures and found to amount to 20.8 h for H727, 27.0 h for UMC-11 and 35.7 h for H835, respectively.
Chemosensitivity tests using clinically established drugs
Cells were exposed to the individual chemotherapeutic drugs in vitro and IC50 values calculated from dose-response relationships following quantitation of the surviving cells by MTT assays. All tests were carried out for four days except assays involving dacarbazine for seven days, since the active metabolite (5-amino-imidazole 4-carboxamide) is slowly formed in vitro. Table 1 shows the results of the chemosensitivity assays for 14 drugs tested against the three carcinoid cell lines. The IC50 values were compared to the following individual achievable peak plasma concentrations (PPC): vinblastine 8.5 ng/ml [22], taxol 3.5±1.4 µM [23], camptothecin derivative topotecan (TPT) 65±20 nM [24], 5-FU 0.6 µM/l [25], doxorubicin 73.5±26.4 ng/ml [26], gemcitabine 50 µM [27], tamoxifen 0.4 µM [28], cisplatin 6.3 µM [29], oxaliplatin 9.1±1.25 µM [30], carboplatin 105 µM [31], mitomycin C 345±151 ng/ml [32], streptozotocin 100±10 µg/ml [33], etoposide 5.6±2.5 µg/ml [34] and dacarbazine 8.6±0.6 µg/ml [35]. UMC-11 cells exhibited marked chemoresistance against most drugs, with the exception of vinblastine, camptothecin, oxaliplatin, carboplatin, mitomycin C and dacarbazine, where IC50 values were below PPC for the respective drugs. The other two permanent cell lines H727 and H835 were sensitive to 10/14 and 9/14, respectively, of the drugs used here. Compared to the H835 cell line, the H727 line that is p53 wildtype was additionally sensitive to dacarbazine. All three cell lines seemed to have a similar sensitivity to oxaliplatin and carboplatin in contrast to their high resistance to cisplatin.
| Drug | Lung carcinoid cell line | Lung carcinoid cell line | Lung carcinoid cell line | Lung carcinoid cell line |
| UMC-11 (IC50) | H727 (IC50) | H835 (IC50) | PPC | |
| Vinblastine ( ng/ml) | 2.0±0.35 | 8.0±2.7 | 2.0±0.3 | 8.5 |
| Taxol (ng/ml) | 10.0±4.4 | 8.0±3.8 | 9.0±3.9 | 1.0 |
| Camptothecin (nM ) | 18.0±5.0 | 0.5±0.1 | 6.5±4.3 | (65) |
| 5-Fluorouracil (µM ) | 30.0±4.6 | 20.0±5.9 | 45.0±8.7 | 0.6 |
| Doxorubicin (nM) | 370.7±72.4 | 24.1±5.2 | 73.1± 2.4 | 73.5 |
| Gemcitabine (nM) | 833.3±103 | 30.0±6.7 | 30.0±20 | 50.0 |
| Tamoxifen (µM) | 9.0±3.3 | 25.0±8.1 | 7.0±2.4 | 0.4 |
| Cisplatin (µM) | 33.3±15 | 83.3±21 | 33.3±7.0 | 6.3 |
| Oxaliplatin (µM ) | 5.0±0.25 | 11.3±5.3 | 6.3±0.51 | 9.1 |
| Carboplatin (µM) | 36.4±7.1 | 3.4±0.4 | 35.8±9.9 | 105 |
| Mitomycin (ng/ml) | 156.0±15 | 10.0±3.0 | 35.0±9.0 | 345.0 |
| Streptozotocin (µg/ml) | 180±33 | 50±21 | 60±19 | 100 |
| Etoposide (µg/ml) | 45.0±12.3 | 0.13±0.03 | 0.25±0.02 | 5.6 |
| Dacarbazine (µg/ml) | 4.2±2.3 | 2.2±1.3 | 16.5±3.9 | 8.6 |
IC50 values are given as mean±SD (n=3), and clinical PPC are shown for comparison
Cytotoxic activity of third-generation platinum drugs in lung carcinoid cell lines
The novel platinum-based drugs satraplatin and picoplatin were tested for cytotoxicity using the three carcinoid cell lines in vitro. In vivo, satraplatin is rapidly converted to JM149 and the highly active metabolite JM118 which were included in this chemosensitivity assay (Fig. 2). The achievable PPCs were 15.9 µM for picoplatin, 1.4 µM for satraplatin and 2.2 µM for JM118, respectively. H835 cells were tested either as a single cell suspension or as globular aggregates with a diameter of approximately 150–250 µM. The chemosensitivity tests showed that picoplatin and JM118 reached IC50 concentrations below their PPCs in H727 and H835 cells, respectively. In addition, H727 proved to be sensitive to JM149. H835 cells grown as multicellular spheroids were more resistant to picoplatin (2.7-fold), JM216 (7.9-fold), JM149 (4.3-fold) and JM118 (8.7-fold).

Cytotoxicity of combinations of platinum-based drugs with DCA in lung carcinoid cell lines
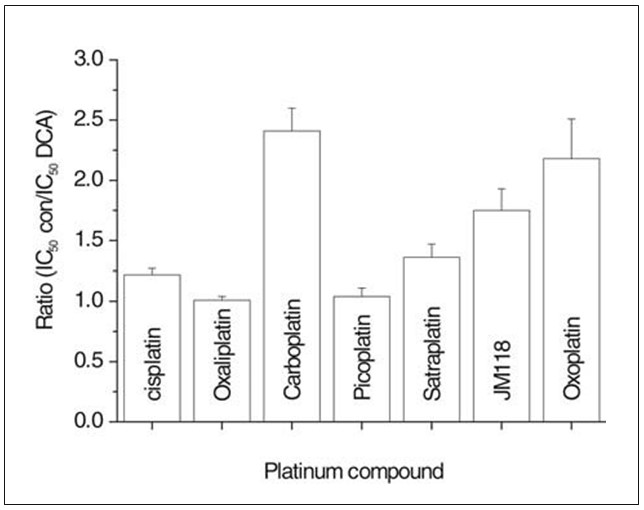
Chemosensitivity assays as described above were furthermore performed using the resistant UMC-11 cell line either in the absence or in continuous presence of 10 mM DCA (Fig. 3). This concentration of DCA reduced proliferation of UMC-11 cells by 22.2±3.2% and lowered IC50 values (sensitisation factor: mean±SD of IC50 of platinum compound in medium alone divided by the IC50 of the respective platinum compound in medium supplemented with 10 mM DCA; IC50 of platinum compound in presence of DCA) for carboplatin (2.41±0.19; 13.1±0.2 µM), satraplatin (1.36±0.11; 7.8±0.4 µM), metabolite JM118 (1.75±0.18; 1.22±0.05 µM) and oxoplatin (2.18±0.33; 4.8±0.14 µM). In contrast, cisplatin (1.22±0.9; 25.3±0.75 µM), oxaliplatin (1.01±0.03; 4.4±0.16 µM) and picoplatin (1.04±0.02; 28.6±0.6 µM) showed low or absent modulation of their cytotoxicity by DCA. Oxoplatin [cis, trans, cis diammine dihydroxido dichlorido platinum(IV)] is currently under investigation as an oral platinum drug.
Discussion
Successful treatment of malignant carcinoid tumours requires a multimodal approach, including resection, tumour reduction by hepatic artery ligation for liver metastases and systemic therapy using cytostatic and/or cytotoxic drugs [1–3, 8]. Octreotide and different long-acting somatostatin analogues yielded biochemical response rates in the range of 30–70%, but objective tumour shrinkage in less than 10% of the patients [36]. Single-agent chemotherapy of carcinoid tumours gave very low response rates that did not exceed 20% for doxorubicin monotherapy [37]. Clinical trials including streptozotocin plus cyclophosphamide or 5-FU did not generate significantly better response rates, ranging between 26 and 33% [18]. In summary, carcinoids can be pharmacologically controlled by treatment with somatostatin analogues and interferon, as well as cytotoxic drugs, such as streptozotocin, dacarbazine, 5-FU, cyclophosphamide, doxorubicin for an extended time; however, the tumours finally progress to a refractory state [3, 16, 38, 39].
Cytotoxicity of a broad range of therapeutics was tested here using the three permanent cell lines UMC-11, H727 and H835 derived from bronchopulmonary carcinoids by Giaccone et al. [40]. H727 and H835 can be regarded mainly as drug-sensitive cells with insensitivity to taxol, 5-FU, cisplatin and tamoxifen. In contrast, UMC-11 cells exhibited marked chemoresistance with the exception of sensitivity to dacarbazine, vinblastine, mitomycin, carboplatin and oxaliplatin. Comparison of the IC50 values of the chemotherapeutics obtained in vitro with the clinically achievable PPCs provides a rough estimate of the expected clinical effi cacy. The in vivo IC50 values of tumour cells may be either reduced under prolonged application of a drug or higher when lower tissue concentrations and diminished tumour cell accessibility are taken into account. Though all three cell lines displayed a similar S-phase fraction in cell cycle distribution measurements, their doubling times differed and amounted to 20.8 and 35.7 h for the chemosensitive lines H727 and H835, respectively, and 27.0 h for chemoresistant UMC-11 cells. According to these data, chemoresistance of the three carcinoid cell lines is not due to differences in their S-phase fraction or doubling times. The in vitro results obtained for streptozotocin, dacarbazine, cisplatin, etoposide, 5-FU, doxorubicin and tamoxifen are in good agreement with the limited clinical activity (<30% response rate) of these drugs in patients with advanced carcinoids [16]. Carboplatin was reported to possess insuffi cient activity in a clinical trial involving carcinoid patients; however, it is not clear whether any of these patients had foregut carcinoids [41]. High expression of the excision repair cross-complementation group 1 (ERCC1) protein, a key component of the repair mechanism for DNA damaged by platinum adducts, in typical carcinoids seemed to be linked to platinum resistance in these patients [42].
Since the development of chemotherapy regimens for advanced carcinoids based on streptozotocin/dacarbazine, 5-FU, cyclophosphamide or others, several new drugs have been introduced and shown to possess significant anticancer activity in related tumours, either as single agents or in combination [3, 43, 44]. These agents include oxaliplatin, a highly active platinum compound, paclitaxel, a taxane targeting polymerisation of microtubuli, analogues of the topoisomerase I inhibitor camptothecin and the antimetabolite gemcitabine. Paclitaxel proved to be less cytotoxic than vinblastine and gemcitabine, which were active against the two sensitive carcinoid cell lines. Paclitaxel was recently evaluated in a clinical study involving 14 patients with carcinoid tumours and nine patients with islet cell tumours and yielded an overall response rate of 8% that was associated with significant haematologic toxicity [45]. The highly active parent compound camptothecin (CPT) was abandoned for clinical use due to high local toxicity upon intravenous application but is currently being reevaluated in novel formulations. Two established derivatives, namely CPT-11 (irinotecan) and topotecan, were modified to obtain higher solubility in water and demonstrated to display clinical activity in a wide range of tumours [46]. The achievable PPCs of approximately 65 nM for topotecan and >70 nM for the active metabolite of CPT-11, SN38, are in the same range as the IC50 value obtained for CPT [32, 47]. A report of chemoresistance in primary cultures of typical carcinoids found relatively high sensitivity to 5-FU, doxorubicin, etoposide and dacarbazine but high resistance to cisplatin, gemcitabine, vinblastine and carboplatin [48]. However, only six out of 60 tumour specimens tested were foregut carcinoids and not every agent was applied to each tumour sample. Therefore, lung carcinoids may exhibit a chemosensitivity pattern distinct to mid- and hindgut tumours, as found in the present study.
Our results show that oxaliplatin is cytotoxic against all three carcinoid cell lines including UMC-11 cells, which display broad drug resistance. Furthermore, clinical response to cisplatin was reported for a single carcinoid patient [49]. The novel platinum complexes satraplatin and picoplatin have not been tested in carcinoid patients so far but showed promising clinical activity in lung cancer trials [20, 50]. UMC-11 cells are insensitive to picoplatin. JM118, the highly active metabolite of satraplatin, showed signifi cant cytotoxicity against all three lung carcinoid cell lines. Sensitivity to the novel platinum compounds is markedly reduced in spheroids of H835 cells compared to single cell suspensions and depends on molecular size and lipophilicity of the respective drug. Therefore, limited access of the chemotherapeutics to carcinoid cells and resulting insufficient drug levels may be associated with drug resistance in carcinoid tumourlets in vivo [49].
Broad resistance to cytotoxic drugs can be attributed to increased suppression of apoptotic tumour cell death via alterations of the characteristics of mitochondria [51]. The metabolism of most solid tumours comprises aerobic glycolysis (Warburg effect), and under these conditions mitochondria fail to provide essential proapoptotic factors. DCA is an orally available small molecule inhibitor of the pyruvate dehydrogenase kinase that increases the flux of pyruvate into mitochondria and thereby favours glucose oxidation over glycolysis [52]. This reverses the suppressed mitochondrial apoptosis in cancer and results in inhibition of tumour growth in vitro and in vivo for breast, prostate and endometrial cancer [53]. In addition to the cellular effects induced by DCA alone it may be capable of enhancing cell death when combined with cytotoxic drugs. DCA had no additive effect in the two non-small-cell lung cancer cell lines A549 and H719 with paclitaxel, etoposide or cisplatin. However, mitaplatin, a compound where two DCA molecules were appended to the axial positions of a six-coordinate Pt(IV) centre, showed promising activity [54, 55]. Our results demonstrate that DCA inhibits growth of the chemoresistant UMC-11 cell line and sensitises the cells to carboplatin, JM118 and oxoplatin at concentrations that can be achieved in vivo [56]. The mechanism of this selectivity of DCA for specific platinum compounds remains to be elucidated. In conclusion, platinum complexes such as oxaliplatin, carboplatin and satraplatin may hold potential for the treatment of lung carcinoids, and the sensitisation of the tumour cells to selected platinum drugs induced by DCA may be of broader applicability in cytotoxic chemotherapy.
Acknowledgment
We wish to thank Dr. Z. Salama for providing platinum complexes and helpful discussions. This project was supported by a grant of the Austrian National Bank (# 13345) to Dr. K. Geissler. Dr. W. Fiebiger and Dr. U. Olszewski PhD contributed equally to this study.
Conflict of interest
The authors declare that they have no conflict of interest relating to the publication of this manuscript.
REFERENCES
1 Öberg K (2003) Diagnosis and treatment of carcinoid tumors. Expert Rev Anticancer Ther 3:863–8772 Modlin IM, Kidd M, Latich I et al (2005) Current status of gastrointestinal carcinoids. Gastroenterology 128:1717–1751
3 Pinchot SN, Holen K, Sippel RS, Chen H (2008) Carcinoid tumors. Oncologist 13:1255–1269
4 Zuetenhorst JM, Taal BG (2005) Metastatic carcinoid tumors: a clinical review. Oncologist 10:123–131
5 Granberg D, Oberg K (2005) Neuroendocrine tumours. Cancer Chemother Biol Response Modif 22:471–483
6 Capella C, Heitz PU, Hofler H et al (1995) Revised classification of neuroendocrine tumors of the lung, pancreas and gut. Virchows Arch 425:547–560
7 Warren WH, Gould VE, Faber LP et al (1985) Neuroendocrine neoplasms of the bronchopulmonary tract: a classification of the spectrum of carcinoid to small cell carcinoma and intervening variants. J Thorac Cardiovasc Surg 89:819–825
8 Plockinger U, Rindi G, Arnold R et al (2004) European Neuroendocrine Tumour Society: guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. A consensus statement on behalf of the European Neuroendocrine Tumour Society (ENETS). Neuroendocrinology 80:394–424
9 Öberg K, Eriksson B (2005) Nuclear medicine in the detection, staging and treatment of gastrointestinal carcinoid tumours. Best Pract Res Clin Endocrinol Metab 19:265–276
10 Dousset B, Saint-Marc O, Pitre J et al (1996) Metastatic endocrine tumors: medical treatment, surgical resection and liver transplantation. World J Surg 20:908–915
11 Raderer M, Kurtaran A, Leimer M et al (2000) Value of peptide receptor scintigraphy using (123) I-vasoactive intestinal peptide and (111)In-DTPAD-Phe1-octreotide in 194 carcinoid patients: Vienna University Experience, 1993 to 1998. J Clin Oncol 18:1331–1336
12 Smolle-Juttner FM, Popper H, Klemen H et al (1993) Clinical features and therapy of “typical” and “atypical” bronchial carcinoid tumors (grade 1 and grade 2 neuroendocrine carcinoma). Eur J Cardiothorac Surg 7:121–125
13 Marty-Ane CH, Costes V, Pujol JL et al (1995) Carcinoid tumors of the lung: do atypical features require aggressive management? Ann Thorac Surg 59:78–83
14 Moertel CG, Sauer WG, Dockerty MB, Baggenstoss AH (1961) Life history of the carcinoid tumor of the small intestine. Cancer 14:901–912
15 Goodwin JD (1975) Carcinoid tumors: an analysis of 2837 cases. Cancer 36:560–569
16 Bertino EM, Confer PD, Colonna JE et al (2009) Pulmonary neuroendocrine/carcinoid tumors: a review article. Cancer 115:4434–4441
17 Moertel CG (1983) Treatment of the carcinoid tumor and the malignant carcinoid syndrome. J Clin Oncol 1:727–740
18 Sun W, Lipsitz S, Catalano P et al (2005) Phase II/III Study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 23:4897–4904
19 Dowell JE (2010) Small cell lung cancer: are we making progress? Am J Med Sci 339:68–76
20 Shah N, Dizon DS (2009) New-generation platinum agents for solid tumors. Future Oncol 5:33–42
21 Dhar S, Lippard SJ (2009) Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proc Natl Acad Sci U S A 106:22199–22204
22 Chong CD, Logothetis CJ, Savaraj N et al (1988) The correlation of vinblastine pharmacokinetics to toxicity in testicular cancer patients. J Clin Pharmacol 28:714–718
23 Rowinsky EK, Burke PJ, Karp JE et al (1989) Phase I and pharmacodynamic study of taxol in refractory acute leukemias. Cancer Res 49:4640–4647
24 Dennis MJ, Beijnen JH, Grochow LB, van Warderdam LJC (1997) An overview of the clinical pharmacology of topotecan. Semin Oncol 24:12–18S
25 Ho DH, Pazdur R, Covington W et al (1998) Comparison of 5-fluorouracil pharmacokinetics in patients receiving continuous 5-fluorouracil infusion and oral uracil plus N1-(2′-tetrahydrofuryl)-5-fluorouracil. Clin Cancer Res 4:2085–2088
26 Wihlm J, Limacher JM, Leveque D et al (1997) Pharmacokinetic profile of high-dose doxorubicin administered during a 6 h intravenous infusion in breast cancer patients. Bull Cancer 84:603–608
27 Touroutoglou N, Gravel D, Raber MN et al (1998) Clinical results of a pharmacodynamically-based strategy for higher dosing of gemcitabine in patients with solid tumors. Ann Oncol 9:1003–1008
28 Kivisto KT, Villikka K, Nyman L et al (1998) Tamoxifen and toremifene concentrations in plasma are greatly decreased by rifampicin. Clin Pharmacol Ther 64:648–654
29 Bonetti A, Franceschi T, Apostoli P et al (1995) Cisplatin pharmacokinetics using a five-day schedule during repeated courses of chemotherapy in germ cell tumors. Ther Drug Monit 17:25–32
30 Graham MA, Lockwood GF, Greenslade D et al (2000) Clinical pharmacokinetics of oxaliplatin: a critical review. Clin Cancer Res 6:1205–1218
31 Oguri S, Sakakibara T, Mase H et al (1988) Clinical pharmacokinetics of carboplatin. J Clin Pharmacol 28:208–215
32 Schilcher RB, Young JD, Ratanatharathorn V et al (1984) Clinical pharmacokinetics of highdose mitomycin C. Cancer Chemother Pharmacol 13:186–190
33 Adolphe AB, Glasofer ED, Troetel WM et al (1975) Fate of streptozotocin (NSC-85998) in patients with advanced cancer. Cancer Chemother Rep 9:547–556
34 Hande K, Messenger M, Wagner J et al (1999) Inter- and intrapatient variability in etoposide kinetics with oral and intravenous drug application. Clin Cancer Res 5:2742–2747
35 Chabot GG, Flaherty LE, Valdivieso M, Baker LH (1990) Alteration of DTIC pharmacokinetics after interleukin-2 administration in melanoma patients. Cancer Chemother Pharmacol 27:157–160
36 Kvols LK, Moertel CG, O’Connell MJ et al (1986) Treatment of the malignant carcinoid evaluation of a long-acting somatostatin analog. N Engl J Med 315:663–666
37 Engstrom PF, Lavin PT, Moertel CG et al (1984) Streptozotocin plus fluorouracil versus doxorubicin therapy for metastatic carcinoid tumor. J Clin Oncol 8:865–890
38 Vilar E, Salazar R, Pérez-García J et al (2007) Chemotherapy and role of the proliferation marker Ki-67 in digestive neuroendocrine tumors. Endocr Relat Cancer 14:221–23
39 García-Yuste M, Matilla JM, Cueto A et al (2007) Typical and atypical carcinoid tumours: analysis of the experience of the Spanish Multi-centric Study of Neuroendocrine Tumours of the Lung. Eur J Cardiothorac Surg 31:192–197
40 Giaccone G, Battey J, Gazdar AF et al (1992) Neuromedin B is present in lung cancer cell lines. Cancer Res 52:2732s–2736s
41 Saltz L, Lauwers G, Wiseberg J, Kelsen D (1993) A phase II trial of carboplatin in patients with advanced APUD tumors. Cancer 72:619–622
42 Skov BG, Holm B, Erreboe A et al (2010) ERCC1 and Ki67 in small cell lung carcinoma and other neuroendocrine tumors of the lung: distribution and impact on survival. J Thorac Oncol 5:453–459
43 Kelly K (2000) New chemotherapy agents for small cell lung cancer. Chest 117:156–162S
44 Teicher BA (2008) Newer cytotoxic agents: attacking cancer broadly. Clin Cancer Res 14:1610–1617
45 Ansell SM, Pitot HC, Burch PA et al (2001) A phase II study of high-dose paclitaxel in patients with advanced neuroendocrine tumors. Cancer 91:1543–1548
46 Iyer L, Ratain MJ (1998) Clinical pharmacology of camptothecins. Cancer Chemother Pharmacol 42:S31–S43
47 Catimel G, Chabot GG, Guastalla JP et al (1995) Phase I and pharmacokinetic study of irinotecan (CPT-11) administered daily for three consecutive days every three weeks in patients with advanced solid tumors. Ann Oncol 6:133–140
48 Lyons JM 3rd, Abergel J, Thomson JL et al (2009) In vitro chemoresistance testing in well-differentiated carcinoid tumors. Ann Surg Oncol 16:649–655
49 Porter AT, Ostrowski MJ (1988) Successful treatment of malignant carcinoid tumour with intravenous cisplatinum. Eur J Surg Oncol 14:703–704
50 Olszewski U, Hamilton G (2010) A better platinum-based anticancer drug yet to come? Anticancer Agents Med Chem 10:293–301
51 Wasilewski M, Scorrano L (2009) The changing shape of mitochondrial apoptosis. Trends Endocrinol Metab 20:287–294
52 Michelakis ED, Webster L, Mackey JR (2008) Dichloroacetate (DCA) as a potential metabolictargeting therapy for cancer. Br J Cancer 99:989–994
53 Sun RC, Fadia M, Dahlstrom JE et al (2010) Reversal of the glycolytic phenotype by dichloroacetate inhibits metastatic breast cancer cell growth in vitro and in vivo. Breast Cancer Res Treat 120:253–260
54 Otterson GA, Wang L, Wu X et al (2008) Effect of dichloroacetate in combination with chemotherapy on human lung cancer cells. J Clin Oncol 26S:14637
55 Dhar S, Lippard SJ (2009) Mitaplatin, a potent fusion of cisplatin and the orphan drug dichloroacetate. Proc Natl Acad Sci U S A 106:22199–22204
56 Li T, Schultz I, Keys DA et al (2008) Quantitative evaluation of dichloroacetic acid kinetics in human: a physiologically based pharmacokinetic modeling investigation. Toxicology 245: 35–48