Zheng Yang1, Kin Y. Tam1
1 Faculty of Health Sciences, University of Macau, Taipa, Macau, China.
Correspondence: Kin Y. Tam
Faculty of Health Sciences, University of Macau, Taipa, Macau, China
Tel.: +853-88224988
Fax: +853-88222314.
Email: [email protected]
Received: 15 April 2016
Revised: 27 July 2016
Accepted: 2 August 2016
Abstract
Glycolysis has been observed as a predominant process for most cancer cells to utilize glucose, which was referred to as “Warburg Effect”. Targeting critical enzymes, such as pyruvate dehydrogenase kinase (PDK) that inversely regulating the process of glycolysis could be a promising approach to work alone or in combination with other treatments for cancer therapy. EGFR inhibitors for Non-Small-Cell Lung Cancer (NSCLC) treatment have been applied for decades in clinical practices with great success, but also their clinical benefits were somewhat hampered by the rising acquired-resistance. Combination drug therapy is an effective strategy to cope with the challenge. In this study, we utilized Dichloroacetate (DCA), a widely regarded PDK inhibitor, together with Erlotinib and Gefitinib, two well-known EGFR inhibitors, and demonstrated that the applications of DCA in combination with either Erlotinib or Gefitinib significantly attenuated the viability of EGFR mutant NSCLC cells (NCI-H1975 and NCI-H1650) in a synergistic manner. This synergistic outcome appears to be a combination effect in promoting apoptosis, rather than co-suppression of either EGFR or PDK signaling pathways. Moreover, we have shown that the combination treatment did not exhibit synergistic effect in other NSCLC cell lines without EGFR mutations (A549 or NCI-H460). Together, these observations suggested that combined targeting of EGFR and PDK in NSCLC cells exerted synergistic effects in an EGFR mutation-dependent fashion.
Keywords: Pyruvate Dehydrogenase Kinase; Dichloroacetate; Epidermal Growth Factor Receptor; Erlotinib; Gefitinib; Drug Combination
Copyright © 2016 Elsevier B.V. All rights reserved.
INTRODUCTION
Lung cancer ranked the first in males and top five in females of newly diagnosed cancer cases and the cancer-related deaths worldwide according to the latest statistics (Jemal et al., 2011), with over 80% of the patients falling into the category of Non-Small-Cell Lung Cancer, or NSCLC (Ke et al., 2015). Traditional strategies for NSCLC treatment often resorted to chemotherapies with either mono-application or combo-administration of platinum-based or other cytotoxic chemicals. However, the objective response rates of these strategies were usually unsatisfactory, with median overall survival usually less than 1 year (Schiller et al., 2002, Pao and Chmielecki, 2010).
EGFR mutation was found in approximate 30% of the NSCLC patients who often responded well to the targeted therapy (Pao and Chmielecki, 2010). This enabled wide applications of small molecular EGFR tyrosine kinase inhibitors (EGFR-TKis), which showed tremendous success in past decades (Hanahan and Weinberg, 2011), as exemplified by Erlotinib and Gefitinib (Dutta and Maity, 2007). However, acquired-resistance usually occurred when patients were treated with EGFR inhibitors, with several identified mechanisms such as original or induced T790M hot spot mutation, activated secondary signaling like MET amplification or PI3K mutation, or conferred epithelial to mesenchymal transition (EMT) (Maione et al., 2015). Disappointingly, combined applications of EGFR inhibitors with chemotherapies brought about higher occurrence of adverse effects, rather than the expected benefit of extending the overall survival of the treated objects (Yan et al., 2015).
Cancer cell metabolism is an emerging field based on the discovery and over half century investigation on the “Warburg Effect” (Ngo et al., 2015), which states that cancer cells tend to metabolize glucose via glycolysis, instead of oxidative phosphorylation, to generate energy (Lu et al., 2015). This phenomenon has driven many studies focusing on key enzymes in glucose metabolism, such as glucose transporters (GLUTs), hexokinase2 (HK2), pyruvate kinase M2 (PKM2), pyruvate dehydrogenase kinase (PDK), lactate dehydrogenase-A (LDHA), and glutaminase, leading to the development of several inhibitors that targeted specific enzymes for anti-cancer therapy (Butler et al., 2013). As a PDK inhibitor, dichloroacetate (DCA) can attenuate cancer progression in many different kinds of cancers through down-regulating pyruvate dehydrogenase phosphorylation (p-PDH) which was controlled by PDK (Kankotia and Stacpoole, 2014). Although several combination studies that involved in the application of DCA for NSCLC treatment have been reported, majority of them were focused on the classical cytotoxic chemotherapy, namely the combined use of DCA and platinum-based drug (Garon et al., 2014, Olszewski et al., 2010). Whether the combination of DCA with EGFR-TKi in EGFR mutated NSCLCs can exert a synergetic effect on anti-cancer therapy remains unknown.
In this study, we demonstrated that combined application of EGFR inhibitors (either Erlotinib or Gefitinib) with DCA synergistically inhibited NCI-H1975 and NCI-H1650 cell growth. Moreover, we explored the possible mechanisms for the combination effects of EGFR and PDK inhibitors. We found that these combinations can only show synergy in NCI-H1975 and NCI-H460, the EGFR mutant NSCLC cell lines, but not in A549 or NCI-H460, the EGFR wild type NSCLC cell lines.
Materials and methods
Cell lines and reagents
NSCLC cell lines, NCI-H1975, NCI-H1650 and A549, were purchased from ATCC, whereas NCI-H460 was a kindly gift from Prof. Thomas Y.C. Leung (Department of Applied Biology and Chemical Technology, Faculty of Applied Science and Textiles, The Hong Kong Polytechnic University). A549 cell was cultured in F-12K/DMEM 1:1 (Gibco) while other cell lines were maintained in RPMI 1640 (Gibco), with supplement of 10% Fetal Bovine Serum (Gibco), in a humidified atmosphere with 5% CO2 at 37 °C.
DCA was purchased from Sigma and dissolved in 1% DMSO in PBS as the stock solution (1.6 M), which was then diluted to various concentrations to form the final working solutions containing 0.1% DMSO in the whole media for cell treatment. Erlotinib and Gefitinib, both from SelleckChem, were initially dissolved in DMSO (Sigma) to form the stock solution with the concentration of 160 mM, and diluted to the individual concentration of working media as that of DCA. Primary antibodies including p-PDH and PDH (Abcam) were from Cell Signaling Technology. α-Tubulin was obtained from Invitrogen.
Cell viability assay
Cell viability for each individual treated or non-treated cells after indicated time of treatment were assessed through MTT assay. Briefly, individual cells for each cell lines were seeded in 96-well plates 24 h before compounds loading. After indicated time period (24 h, 48 h and 72 h, respectively), culturing media with compounds were discarded and 100 μl fresh whole media containing 0.5 mg/ml MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide, Sigma) was added. After 4 h of incubation at 37 °C, the solvent was removed and 100 μl DMSO was added to each well, with the aid of gentle shaking to dissolve the formazan crystals. O.D. value of each well was measured at 570 nm through SpectraMax M5 Microplate Reader (Molecular Devices).
Combination index (CI) calculation
The CI value was enrolled for synergistic evaluation of cell viability between the combination and each of the single groups, which calculated as a function of fraction of cancer cells affected (Fa) based on the Chou-Talalay equation (Chou and Talalay, 1984): CI=(D)1/(Dx)1+(D)2/(Dx)2, where (D)1 and (D)2 indicates the doses applied to achieve a necessary response in combination, and (Dx) refers to individual drug doses needed to achieve similar response. Analyses of the CI values were performed using the CalcuSyn software (Biosoft), with CI<1, CI=1 and CI>1 indicate synergistic, additive and antagonistic effects, respectively.
Colony formation assay
All of the four NSCLC cell lines were seeded in 6-well plates with each well contained 200–800 cells in 2 ml media. Media with either individual compounds or in combination was added 24 h after cells were inoculated for a continuous 3 days treatment, and then replaced by drug-free media every 3 days. 15 days after cell plating, cell colonies were fixed in 95% ethanol for 15 min, stained by 0.1% Crystal Violet (Sigma) and dried. Colonies with over 100 cells were counted as positive.
Western blotting assay
Both NCI-H1975 and A549 cells were seeded in 6-well plates and treated with media containing mono-compound or combination for defined time periods. Treated cells were incubated in cell lysate buffer (Cell Signaling Technology) with gently shaking for 15 min, and then centrifuged at 12,000 rpm/min at 4 °C for another 15 min. Protein concentration of each sample in the supernatant was assessed by Pierce® BCA Protein Assay Kit (Thermo) and balanced to the same level, followed by a 8 min protein denaturation with SDS loading buffer at 100 °C. Proteins in the sample were separated by SDS-PAGE electrophoresis, transferred onto nitrocellulose filter membrane (Whatman), blocked in 5% fat-free milk for 2 h, breezed with desired primary antibodies overnight, followed with secondary HRP-linked anti-rabbit or anti-mouse IgG (Cell Signaling Technology) for 2 h. Membranes were finally scanned in a Chemidoc® MP Imaging System (Bio-Rad) after 2 min incubation in Clarity Western ECL Substrate (Bio-Rad).
Flow cytometry assay
NCI-H1975 and A549 cells were seeded and treated as described in previous section. Treated cells were detached from the bottom of plates with Trypsin-EDTA (Gibco) and washed with PBS for two times. Apoptotic detections were performed using the FITC Annexin-V Apoptotic Detection Kit (Biolegend). In brief, harvested cell samples were suspended in 500 μl Binding Buffer containing 5 μl of Annexin V-FITC and 10 μl of Propidium Iodide (PI) for 15 min before FACS analysis on an Accuri C6 Flow Cytometer (BD). Cell samples for mitochondrial membrane potential (MMP) measurement were incubated in 1 ml whole media containing 2 μM JC-1 (5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolycarcocyanine iodide, Sigma) for 15 min, followed by one wash and re-suspension in PBS for FACS analysis.
Statistical analysis
All data were presented as the Mean±S.D. IC50 of individual compounds at indicated time point were performed by GraphPad 5.1 (Prism). Statistical comparisons between different sample groups were performed through Excel 2010 by using the Student’s t test. A P value of less than 0.05 was considered as statistically significant.
Results
Erlotinib, Gefitinib and DCA attenuated cell proliferation in NCI-H1975 and A549 NSCLC cell lines
We sought to determine in vitro anti-cell proliferation effects of the three selected compounds in NCI-H1975, NCI-H1650, A549 and NCI-H460 NSCLC cell lines. Cells were treated with decreased 2-fold series dilution of each compounds for 24 h, 48 h or 72 h, respectively. Cell viabilities were assessed by MTT assay. As shown in Fig. 1, Erlotinib, Gefitinib or DCA led to a significant decrease in cell viability in all of the four cell lines in a dose- and time-dependent manner. It can be seen that the data variabilities in IC50 appear to be lower in 72 h for most of the cell lines (see Table 1). From here on, we thus selected 72 h as the end point for the combination treatment.
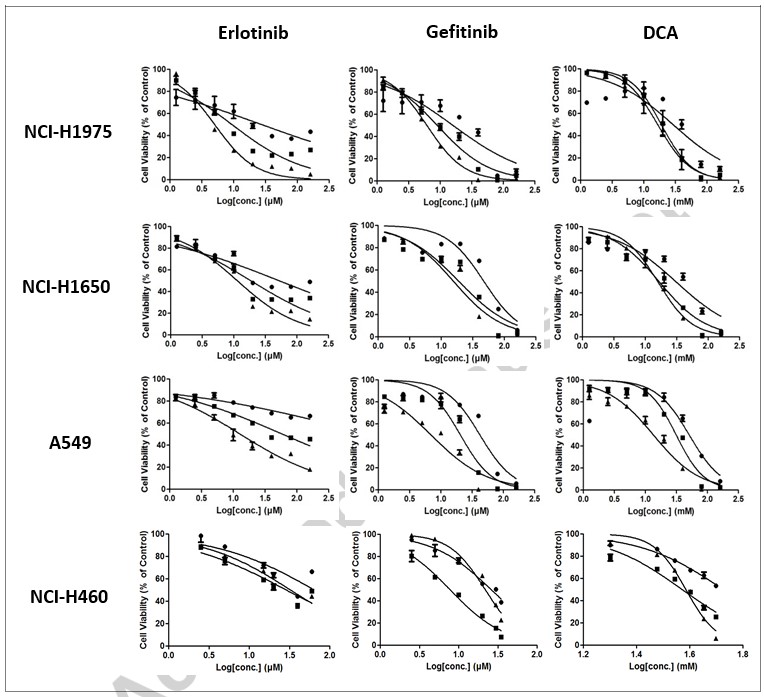
| NCI-H1975 | NCI-H1650 | A549 | NCI-H460 | ||
| Erlotinib (μM) | 24 h | 27.33±15.06 | 52.69±0.80 | N/A | N/A |
| 48 h | 9.45±1.48 | 18.67±0.66 | 59.66±3.06 | 28.72±3.29 | |
| 72 h | 5.04±0.01 | 11.42±0.10 | 13.14±3.70 | 32.66±3.32 | |
| Gefitinib (μM) | 24 h | 16.22±8.37 | 47.30±3.98 | 41.96±3.38 | 26.19±1.98 |
| 48 h | 9.09±1.91 | 18.97±0.62 | 20.66±2.95 | 7.74±0.37 | |
| 72 h | 6.15±0.18 | 15.61±1.15 | 6.97±0.22 | 21.86±0.09 | |
| DCA (mM) | 24 h | 29.51±3.25 | 31.09±4.10 | 50.25±6.89 | N/A |
| 48 h | 19.56±6.31 | 16.84±4.53 | 31.35±1.00 | 37.26±0.47 | |
| 72 h | 16.60±4.46 | 15.81±0.26 | 13.55±2.32 | 38.47±0.35 |
Combined strategies suppressed cell viability synergistically and inhibited colony formation significantly in NCI-H1975 and NCI-H1650, but not work as well in A549 and NCI-H460
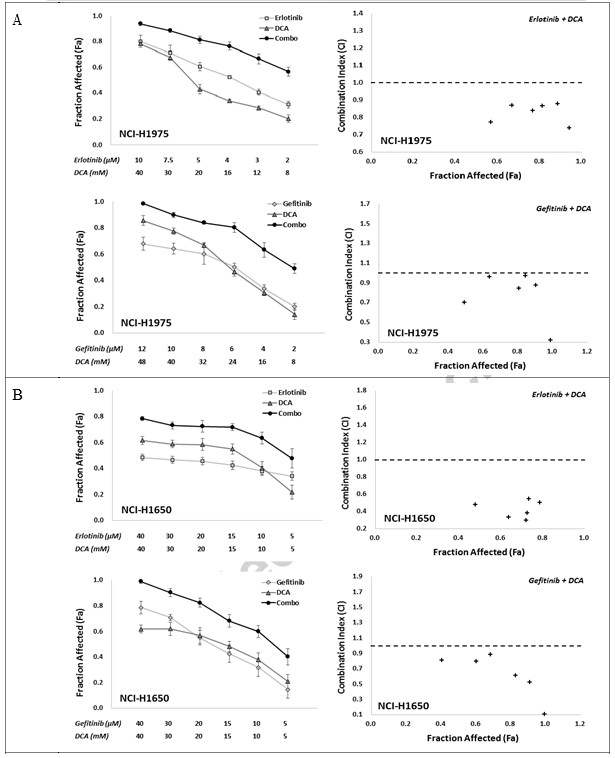
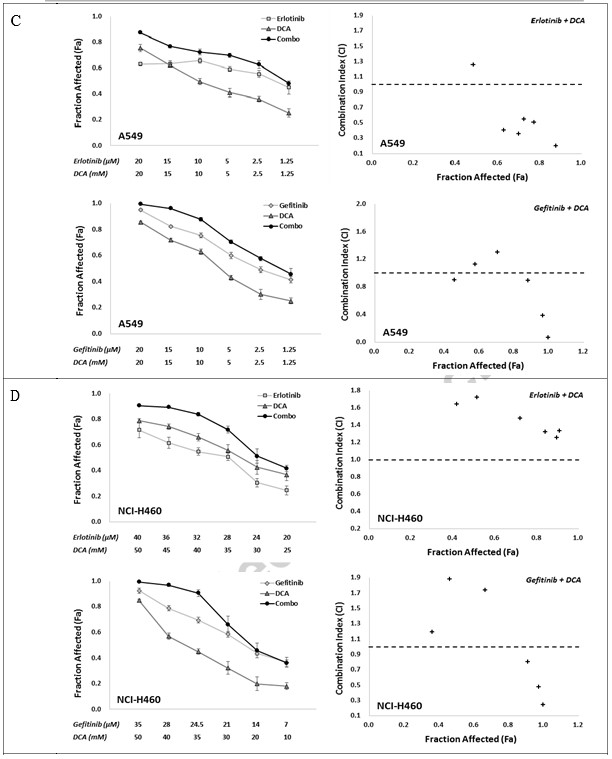
To determine in vitro synergistic response to the combination of EGFR inhibitors with DCA, we utilized two combo strategies, namely Erlotinib with DCA and Gefitinib with DCA, with six individual treating concentration points for each compound, which conferred anti-cell viability between around IC80 to IC20 in a fixed ratio. The combination effects were evaluated using MTT assay. As shown in Fig. 2, both of the two combinations, Erlotinib with DCA and Gefitinib with DCA, clearly exhibited synergy in NCI-H1975 and NCI-H1650 cell lines, where the CI values in all the combined groups were lower than 1 (Fig. 2A and B). For A549 and NCI-H460 cell line, although all the combination groups in two strategies showed elevated Fa value in comparison with their mono-applications, the CI values of some combination groups were above 1 (Fig. 2C and D), suggesting the combination strategies in A549 and NCI-H460 did not work as well as in NCI-H1975 and NCI-H1650.
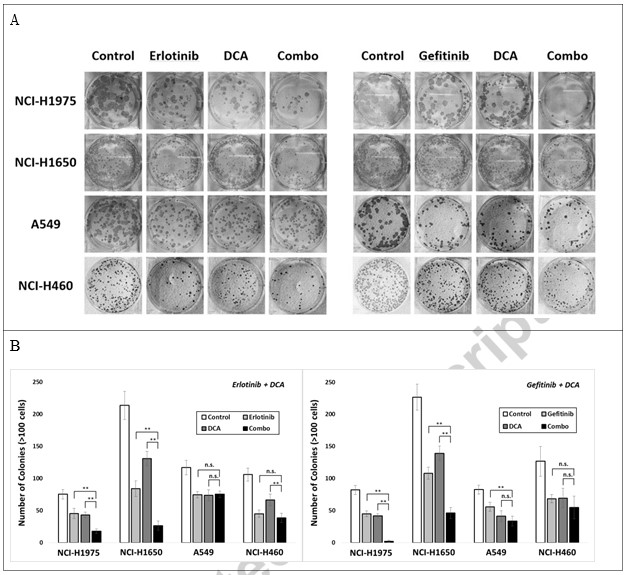
To further explore whether the combination can attenuate cancer cell colony formation, we seeded those four cell lines in 6-well plate, and treated with either Erlotinib, Gefitinib or DCA at concentrations achieving around 40% colonies inhibition for 3 days, or their combinations. 15 days after cell plating, colonies with over 100 cells were counted, with data depicted in Fig. 3. Combination treatment of Erlotinib with DCA significantly repressed colony formation in NCI-H1975 and NCI-H1650, but not in A549 and NCI-H460, indicating that the combination strategy may work only in NCI-H1975 and NCI-H1650, the EGFR mutant NSCLC cells.
Erlotinib/Gefitinib and DCA acted independently in their targeted signaling pathways in NCI-H1975 cell line
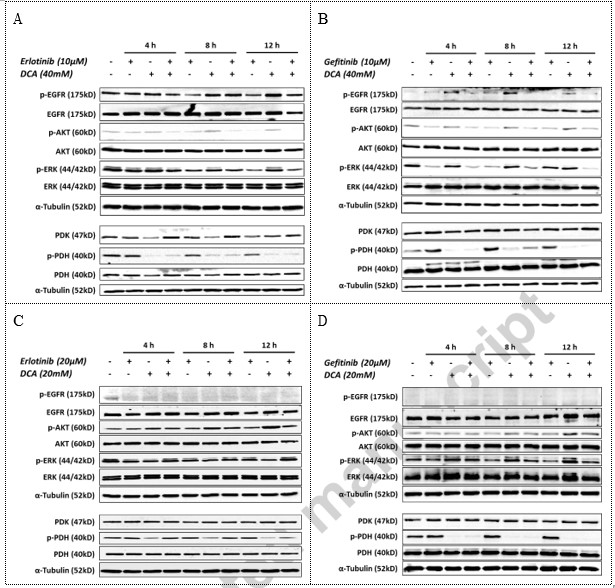
To investigate the possible effect of the combination in signaling pathways, we performed western blotting analysis to study the relevant phosphorylation events in NCI-H1975 and A549 NSCLC cells at defined time points during combination drug treatment. As shown in Fig. 4, in NCI-H1975 EGFR mutant cell line, Erlotinib or Gefitinib suppressed the level of phosphorylated EGFR as well as phosphorylated AKT and phosphorylated ERK1/2, two key proteins that situated in the downstream of EGFR signaling, but did not decrease the level of phosphorylated PDH. DCA, on the other hand, inhibited PDH phosphorylation significantly, but failed to decrease the level of phosphorylated key proteins in EGFR signaling in NCI-H1975 (Fig. 4A and B). However in A549 EGFR wild type NSCLC cells, Erlotinib and Gefitinib did not affect the downstream signaling of EGFR, e.g. the phosphorylated AKT and phosphorylated ERK, whereas DCA significantly attenuated phosphorylated PDH (Fig. 4C and D). Interestingly, it seemed that DCA can activated AKT through elevation its level of phosphorylation over time in both of the two independent studies (Fig. 4C and D). These data suggested that Erlotinib and DCA worked independently on their respective signaling pathway.
Combination of Erlotinib and DCA significantly enhanced cell apoptosis in NCI-H1975, but not in A549
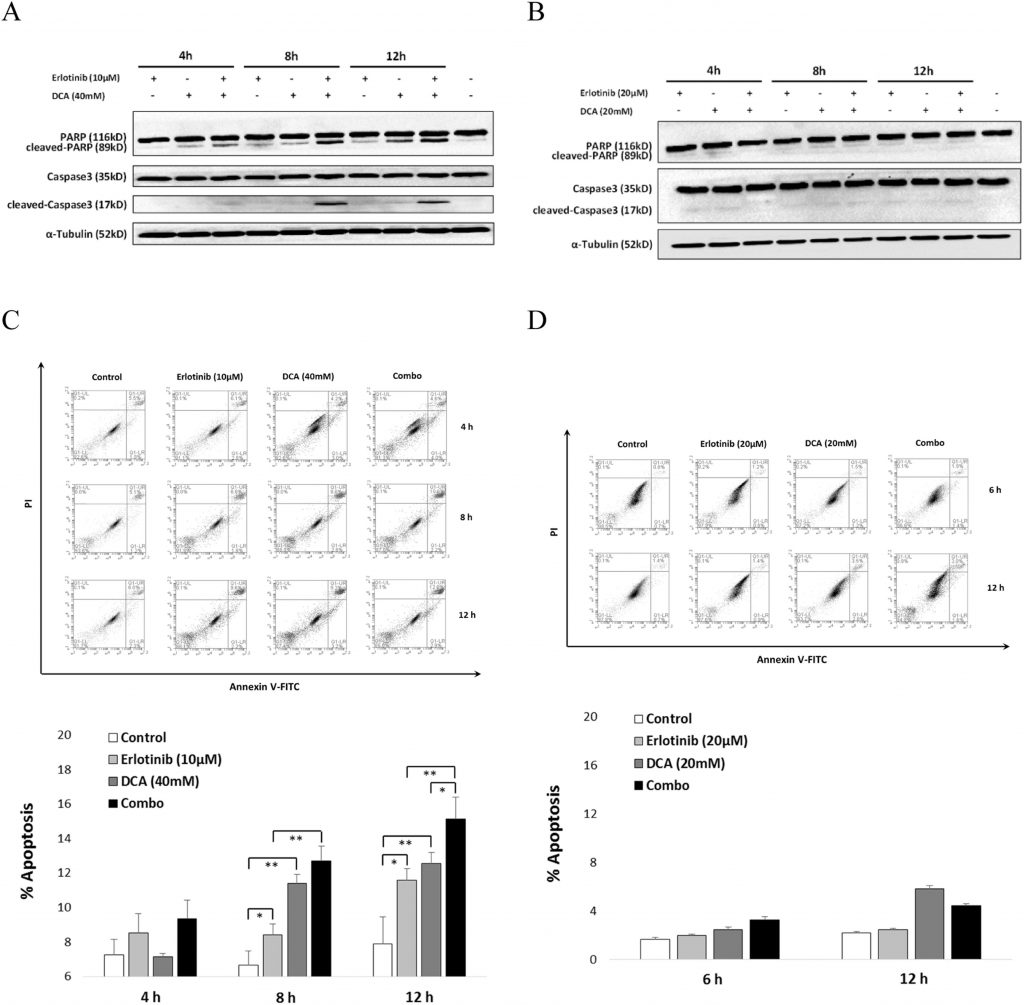
To account for the synergistic effect of Erlotinib and DCA on cell viability, we interrogate whether they collaboratively promoted cell apoptosis. As shown in Fig. 5, western blotting results demonstrated that the combination led to significant activation of Caspase3 and its substrate, cleaved-PARP, after 8 h treatment in NCI-H1975 (Fig. 5A), while no obvious activation of Caspase3 or PARP was observed in A549 (Fig. 5B). Further FACS analysis showed that Erlotinib combined with DCA promoted a relevant larger portion of NCI-H1975 cell apoptosis significantly than each compound alone after cells were treated for 12 h (Fig. 5C), while the combination failed to induce cell apoptosis in A549 cell either in each of the mono-use or in combined manner (Fig. 5D). These results indicated combination boost of cell apoptotic induction by Erotinib and DCA is viable only in NCI-H1975, the EGFR mutant cells, but not in A549, the EGFR wild type NSCLC cells.
MMP decreased significantly in combo strategy than in mono therapy in NCI-H1975
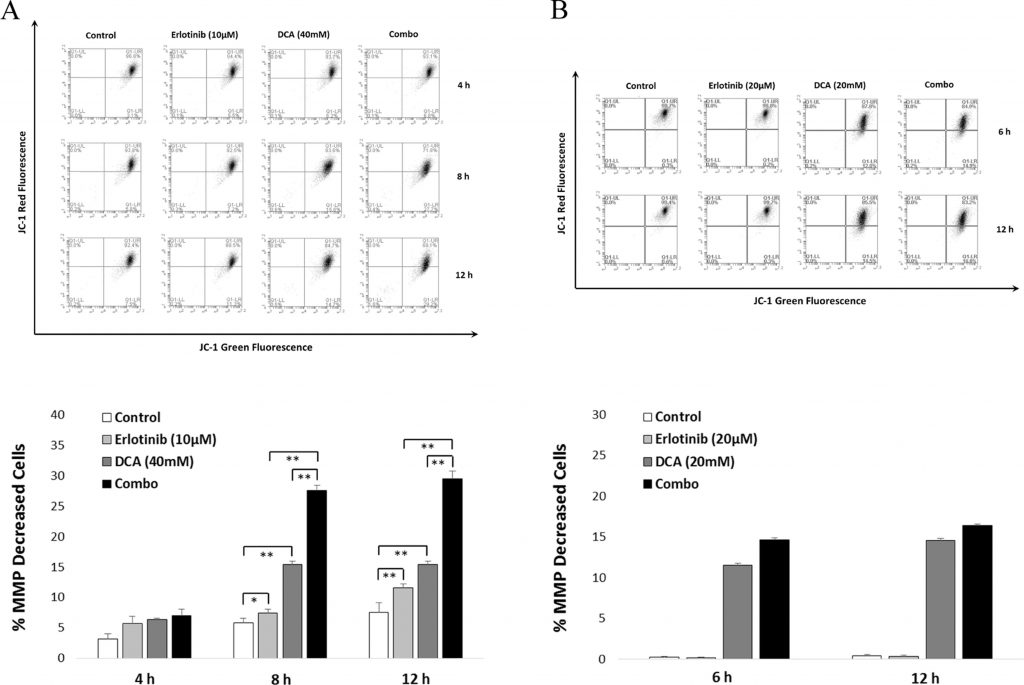
To further confirm the significant effect of the combination treatment to cell apoptosis, we utilized JC-1 assay to determine whether the treatment can lead to a decrease in MMP, one of the hallmark of cell apoptosis. As shown in Fig. 6, the FACS data were consistent with the cell apoptosis results depicted in Section 3.4. It can be seen that the combination demonstrated significance in MMP decrease after both 8 and 12 h treatment in comparison with either mono-application of Erlotinib or DCA in NCI-H1975 cells (see Fig. 6A). For A549 cells, MMP decrease can be detected only when DCA was applied (see Fig. 6B).
Discussion
To test our hypothesis that the combination of EGFR inhibitors with PDK inhibitor, DCA, could exert anti-cancer effect synergistically in NSCLC cells, we applied MTT assay for cell viability evaluation. Results obtained from these study were in good accordance with the hypothesis. Specifically, we have showed that EGFR inhibitors (Erlotinib or Gefitinib) together with DCA synergistically attenuated NCI-H1975 and NCI-H1650 cell viability with all CI values <1 at different dose levels of compound combinations as well as significantly decreased cancer cell colony formation (Fig. 2, Fig. 3). Meanwhile, these synergistic effects did not show up in A549 or NCI-H460 cell lines (EGFR wild type NSCLC cell lines), indicating combination strategy probably suppress cell progression in a synergistic way only in NSCLCs that harbored EGFR mutation.
Velpula et al. (2013) reported that either administration of Erlotinib or Gefitinib attenuated both p-EGFR and PDK1 expression level in U251 and 5310, while application of DCA in these two cells decreased expression of p-EGFR and PDK1 as well. It is plausible that the combination effect of EGFR and PDK inhibitors could be attributed to the co-suppression of either EGFR or PDK signaling. With this notion in mind, we carried out western blotting analysis to evaluate the protein level alterations in those two signal pathways. It has been shown that both Erlotinib and Gefitinib inhibited the phosphorylation of EGFR as well as the phosphorylation of two of its classic down-stream signaling proteins, namely AKT and ERK. Meanwhile, although PDK inhibitor DCA attenuated PDK1 expression slightly, the phosphorylation of PDH, a key enzyme responsible for converting pyruvate into Acetyl-CoA for citric acid cycle instead of launching glycolysis (Kankotia and Stacpoole, 2014), was significantly suppressed when DCA applied (Fig. 4). Apparently, Erlotinib/Gefitinib and DCA worked neither in a crosstalk manner nor in an additive inhibitory effect on each other’s signaling in NCI-H1975 cells. Our data suggested that the synergistic anti-cancer effect of the drug combination treatment in NCI-H1975 is not solely dependent on either EGFR or PDK signaling pathways.
To find other possible mechanisms of the combination effect, we turned our attention to the induction of apoptosis. It was reported that Erlotinib is able to induce NCI-H1975 cell apoptosis (Nie et al., 2015), and DCA also conferred cell apoptosis in several different cancer cells (Madhok et al., 2010, Wong et al., 2008). As the cleavage of Caspase3 and its target cleaving substrate PARP are two elements involved in cell apoptosis (Zhang et al., 2015), we first examined the activation level of Caspase3 and PARP in NCI-H1975 when treated with either Erlotinib or DCA alone as well as the combination. Our results suggested that cleaved PARP of 89 kDa size increased in a time-dependent manner when either Erlotinib or DCA treated alone. Importantly, the combination treatment showed significantly higher activity of PARP when comparing with compounds applied alone. Expression of cleaved Caspase3 at 17 kDa, which peaked at 8 h, presented a sharp increase when NCI-H1975 cell was treated with the combination of Erlotinib and DCA, (Fig. 5A). These results indicated that the combination effect was likely due to the additive effect in promoting cell apoptosis. It is further confirmed by flow cytometry analysis of Annexin-V and PI double stained cell. These observations were consistent with Caspase3 and PARP activation analysis, in which the combination treatment led to a moderate but distinguishable induction of cell apoptosis when NCI-H1975 cell was treated for 12 h as compared with either Erlotinib or DCA application alone (Fig. 5B). However, neither activated Caspase3/PARP nor synergistic induction of cell apoptosis were observed in A549 cell line (Fig. 5A and B), suggesting the promotion of apoptosis could be the possible mechanism for the combination effect of Erlotinib and DCA on EGFR mutant NSCLC cells.
The process of cell apoptosis could be divided into a series of stages, such as the change of cell morphology, loss of mitochondrial membrane potential, permeability alteration, DNA fragmentation and so on (Fiandalo and Kyprianou, 2012). Decrease of MMP was reported by several studies as the consequence of DCA treatment in cancer cells (Emadi et al., 2015). Previous study have demonstrated that Erlotinib led to the loss of MMP in NSCLCs (Qian et al., 2009). In order to investigate whether the combination can affect MMP in NCI-H1975 significantly, we harnessed mitoprobe JC-1 assay to evaluate the level of MMP after treatment by Erlotinib, DCA, or the combination. As shown in Fig. 6, the combination led to a significant decrease of MMP in post 8 and 12 h of treatment in NCI-H1975 cells (but not in A549 cells), suggesting the combination effect of induced cell apoptosis is associated with the depolarization of mitochondrial membrane potential.
In summary, we have demonstrated that the combined use of EGFR inhibitor, namely, Erlotinib or Gefitinib, and PDK inhibitor, DCA, showed synergistic anti-cancer effect in NCI-H1975 and NCI-H1650 cells. Collaborative promotion of cell apoptosis has been identified as one of the probable mechanisms of the combination effect. In particular, the combined application of Erlotinib and DCA not only activated Caspase3 and PARP but also decreased MMP significantly. Moreover, our results indicated that the combination effect might only be apparent in NSCLC cells with EGFR mutations based on our present results. Further evaluations are being undertaken in our laboratory to confirm this observation in more NSCLC cell lines.
Conflict of interest
The authors declare no conflict of interest for this article.
Role of the funding source
This work was supported by the Science and Technology Development Fund, Macao S.A.R (FDCT) (Project reference no. 086/2014/A2) and the University of Macau (Grant no. MRG021-TKY-2015-FHS).
Acknowledgments
We thank the financial support from the Science and Technology Development Fund, Macao S.A.R (FDCT) (Project reference no. 086/2014/A2) and the University of Macau (Grant no. MRG021-TKY-2015-FHS). We thank Prof. Thomas Y.C. Leung (PolyU, HK) for samples of NCI-H460 cells. Thanks are due to Dr. Xiaohui Hu for helpful discussion and proof-reading the manuscript.
REFERENCES
1 Butler et al., 2013 E.B. Butler, Y. Zhao, C. Muñoz-Pinedo, J. Lu, M. Tan Stalling the engine of resistance: targeting cancer metabolism to overcome therapeutic resistance Cancer Res., 73 (2013), pp. 2709-27172 Chou and Talalay, 1984 T.C. Chou, P. Talalay Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors Adv. Enzym. Regul., 22 (1984), pp. 27-55
3 Dutta and Maity, 2007 P.R. Dutta, A. Maity Cellular responses to EGFR inhibitors and their relevance to cancer therapy Cancer Lett., 254 (2007), pp. 165-177
4 Emadi et al., 2015 A. Emadi, M. Sadowska, B. Carter-Cooper, V. Bhatnagar, I. van der Merwe, M.J. Levis, E.A. Sausville, R.G. Lapidus Perturbation of cellular oxidative state induced by dichloroacetate and arsenic trioxide for treatment of acute myeloid leukemia Leuk. Res., 39 (2015), pp. 719-729
5 Fiandalo and Kyprianou, 2012 M.V. Fiandalo, N. Kyprianou Caspase control: protagonists of cancer cell apoptosis Exp. Oncol., 34 (2012), pp. 165-175
6 Garon et al., 2014 E.B. Garon, H.R. Christofk, W. Hosmer, C.D. Britten, A. Bahng, M.J. Crabtree, C.S. Hong, N. Kamranpour, S. Pitts, F. Kabbinavar, C. Patel, E. von Euw, A. Black, E.D. Michelakis, S.M. Dubinett, D.J. Slamon Dichloroacetate should be considered with platinum-based chemotherapy in hypoxic tumors rather than as a single agent in advanced non-small cell lung cancer J. Cancer Res. Clin. Oncol., 140 (2014), pp. 443-452
7 Hanahan and Weinberg, 2011 D. Hanahan, R.A. Weinberg Hallmarks of cancer: the next generation Cell, 144 (2011), pp. 646-674
8 Jemal et al., 2011 A. Jemal, F. Bray, M.M. Center, J. Ferlay, E. Ward, D. Forman Global cancer statistics CA: Cancer J. Clin., 61 (2011), pp. 69-90
9 Kankotia and Stacpoole, 2014 S. Kankotia, P.W. Stacpoole Dichloroacetate and cancer: new home for an orphan drug? Biochim. Biophys. Acta, 1846 (2014), pp. 617-629
10 Ke et al., 2015 E.E. Ke, Q. Zhou, Y.L. Wu Emerging paradigms in targeted treatments for Asian patients with NSCLC Expert Opin. Pharmacother., 16 (2015), pp. 1167-1176
11 Lu et al., 2015 J. Lu, M. Tan, Q. Cai The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism Cancer Lett., 356 (2015), pp. 156-164
12 Madhok et al., 2010 B.M. Madhok, S. Yeluri, S.L. Perry, T.A. Hughes, D.G. Jayne Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells Br. J. Cancer, 102 (2010), pp. 1746-1752
13 Maione et al., 2015 P. Maione, P.C. Sacco, A. Sgambato, F. Casaluce, A. Rossi, C. Gridelli Overcoming resistance to targeted therapies in NSCLC: current approaches and clinical application Ther. Adv. Med. Oncol., 7 (2015), pp. 263-273
14 Ngo et al., 2015 H. Ngo, S.M. Tortorella, K. Ververis, T.C. Karagiannis The Warburg effect: molecular aspects and therapeutic possibilities Mol. Biol. Rep., 42 (2015), pp. 825-834
15 Nie et al., 2015 P. Nie, W. Hu, T. Zhang, Y. Yang, B. Hou, Z. Zou Synergistic induction of erlotinib-mediated apoptosis by resveratrol in human non-small-cell lung cancer cells by down-regulating survivin and up-regulating PUMA Cell Physiol. Biochem., 35 (2015), pp. 2255-2271
16 Olszewski et al., 2010 U. Olszewski, T.T. Poulsen, E. Ulsperger, H.S. Poulsen, K. Geissler, G. Hamilton In vitro cytotoxicity of combinations of dichloroacetate with anticancer platinum compounds Clin. Pharmacol., 2 (2010), pp. 177-183
17 Pao and Chmielecki, 2010 W. Pao, J. Chmielecki Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer Nat. Rev. Cancer, 10 (2010), pp. 760-774
18 Qian et al., 2009 X. Qian, J. Li, J. Ding, Z. Wang, W. Zhang, G. Hu Erlotinib activates mitochondrial death pathways related to the production of reactive oxygen species in the human non-small cell lung cancer cell line A549 Clin. Exp. Pharmacol. Physiol., 36 (2009), pp. 487-494
19 Schiller et al., 2002 J.H. Schiller, D. Harrington, C.P. Belani, C. Langer, A. Sandler, J. Krook, J. Zhu, D.H. Johnson Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer N. Engl. J. Med., 346 (2002), pp. 92-98
20 Velpula et al., 2013 K.K. Velpula, A. Bhasin, S. Asuthkar, A.J. Tsung Combined targeting of PDK1 and EGFR triggers regression of glioblastoma by reversing the Warburg effect Cancer Res., 73 (2013), pp. 7277-7289
21 Wong et al., 2008 J.Y. Wong, G.S. Huggins, M. Debidda, N.C. Munshi, I. De Vivo Dichloroacetate induces apoptosis in endometrial cancer cells Gynecol. Oncol., 109 (2008), pp. 394-402
22 Yan et al., 2015 H. Yan, H. Li, Q. Li, P. Zhao, W. Wang, B. Cao The efficacy of synchronous combination of chemotherapy and EGFR TKIs for the first-line treatment of NSCLC: a systematic analysis PLoS One, 10 (2015), p. e0135829
23 Zhang et al., 2015 L. Zhang, F. Dai, P.L. Sheng, Z.Q. Chen, Q.P. Xu, Y.Q. Guo Resveratrol analogue 3,4,4′-trihydroxy-trans-stilbene induces apoptosis and autophagy in human non-small-cell lung cancer cells in vitro Acta Pharmacol. Sin., 36 (2015), pp. 1256-1265
Related content: