Akbar Khan, MD; Denis Marier, ND; Eric Marsden, ND; Douglas Andrews, ND; Isaac Eliaz, MD
Akbar Khan, MD, is the medical director of Medicor Cancer Centres, Inc, in Toronto, Ontario, Canada. Denis Marier, ND, is the director of Canadian Clinic for Integrative Medicine in Windsor, Ontario. Eric Marsden, ND, is the director of Marsden Center of Naturopathic Excellence in Maple, Ontario. Douglas Andrews, ND, is a staff naturopathic doctor at Medicor Cancer Centres, Inc. Isaac Eliaz, MD, is the director of Amitabha Medical Clinic, in Santa Rosa, California.
Corresponding author: Akbar Khan, MD E-mail address: [email protected]
Abstract
Oral dichloroacetate sodium (DCA) is currently under investigation as a single agent and as an adjuvant for treatment of various cancers. One of the factors limiting its clinical use in a continuous oral regimen is a doserelated, reversible neurotoxicity, including peripheral neuropathy and encephalopathy. The intravenous (IV) route has a number of potential advantages, including (1) pulsed dosing to achieve higher concentrations than feasible with oral use, (2) a longer washout period to reduce the potential for neurotoxicity, and (3) a bypassing of the digestive system, which is particularly significant for advanced-stage cancer patients. Data were available on high-dose IV DCA (up to 100 mg/kg/dose) that have confirmed its safety, both in healthy volunteers and in critically ill patients, allowing the authors to begin offlabel treatment of cancer patients. In several of their patients treated with IV DCA, the authors observed clinical, hematological, or radiological responses. This article presents 3 cases with patients who had recurrent cancers and for whom all conventional therapies had failed: (1) a 79-y-old male patient with colon cancer who had liver metastases, (2) a 43-y-old male patient with angiosarcoma who had pancreatic and bone metastases, and (3) a 10-y-old male patient with pancreatic neuroendocrine carcinoma who had liver metastases. (Altern Ther Health Med. 2014;20(suppl 2):21-28.)
Oral sodium dichloroacetate (DCA) is a drug that is currently under investigation as a single agent and an adjunctive cancer treatment.1 As of this writing, an ongoing phase I trial of oral DCA for recurrent or metastatic solid tumors is occurring at the University of Alberta and 2 trials of oral DCA for head and neck cancers are occurring at Stanford University.
DCA has been extensively studied by Stacpoole2-5 for the treatment of congenital lactic acidosis, which includes a group of inherited mitochondrial diseases. The safety profile for use of oral DCA in humans has been established through this body of work. The drug has been found to be relatively safe, with no hematologic, cardiac, pulmonary, or renal toxicity.6 The main toxicity is neurological, primarily peripheral neuropathy, and this condition is reversible.7 DCA-induced delirium has been observed and is rapidly reversible upon discontinuation of the drug.8 An asymptomatic but reversible elevation of liver enzymes can occur in a small percentage of patients.9
In January 2007, Bonnet et al10 published a groundbreaking paper that demonstrated that DCA was effective in treating human breast, lung, and brain cancers in vitro and in vivo (in rats) by novel metabolic pathways that involve inhibition of mitochondrial pyruvate dehydrogenase kinase. The researchers reported that DCA triggered apoptosis selectively in cancer cells by reducing mitochondrial membrane potential, blocking aerobic glycolysis (Warburg effect), and activating mitochondrial potassium-ion channels. Subsequently, DCA has been further studied and found to have anticancer activity for multiple cancer types, including colon,11 prostate,12 ovarian,13 neuroblastoma,14 lungcarcinoid,15 cervical,16 endometrial,17 cholangiocarcinoma,18 sarcoma19 and T-cell–lymphoma cancers.20
Other mechanisms of DCA action against cancer cells have also been proposed. These methods include (1) inhibition of angiogenesis,21 (2) alteration of expression of hypoxia-inducible factor 1-α (HIF1-α),21 and (3) alteration of pH regulators vacuolar-type H+-ATPase (V-ATPase) and monocarboxylate transporter 1 (MCT1) and other regulators of cell survival, such as p53-upregulated modulator of apoptosis (PUMA), glucose transporter 1 (GLUT1), B-cell lymphoma 2 (BCL2) protein, and cellular tumor antigen p53 (p53).20 However, some studies have had different results, showing decreased apoptosis in certain cancer-cell lines under hypoxic conditions.22,23
DCA has also been shown in vitro to increase the cytotoxicity of selected platinum compounds, suggesting a potential for clinical application in platinum-resistant, small-cell lung cancer; Ewing’s sarcoma; and ovarian cancer.24 Another in vitro study found significant, synergistic, antiproliferative effects using cervical-cancer cell lines with a combination of DCA and cisplatin.25 The body of literature clearly shows potential for further research and development of DCA.
Based on original work by Bonnet et al,10 one of the current authors began to use oral DCA in 2007 as an off-label treatment for cancer patients who had a poor prognosis or had failed to respond to conventional cancer therapies. Peripheral neuropathy26 was observed to be the main factor limiting clinical utility. This author developed a DCA protocol to address the neuropathy in collaboration with another of the authors, a naturopathic physician. This work resulted in an oral-DCA regimen that included the natural neuroprotective medications acetyl-L-carnitine,27-30 R-α-lipoic acid,31-34 and benfotiamine.35-37 Clinical observation of more than 300 advanced-cancer patients treated with this regimen revealed that 60% to 70% exhibited measurable benefits from DCA. The approximate risk of neuropathy was 20%, with a 20- to 25-mg/kg/day dose of DCA that was on a 2-weeks-on/1-week-off cycle, in conjunction with the 3 natural supplements.
The intravenous (IV) route has a number of therapeutic advantages, including (1) higher blood levels because pulsed IV dosing can achieve a higher concentration than is feasible with an oral dosage, (2) a longer washout period to reduce the potential for neurotoxicity, and (3) a bypassing of the digestive system, which is particularly significant for advanced-stage cancer patients. Data were available on highdose, IV DCA up to 100 mg/kg/dose that have confirmed its safety, both in healthy volunteers38 and in critically ill patients.39 Authors Eliaz and Khan developed an IV-DCA treatment protocol and translated it into clinical practice. Khan had prior experience, starting in 2007, with IV DCA, using it to treat patients with complete bowel obstruction. However, it was not possible in Khan’s earlier use of IV DCA dating back to 2007 to assess long-term responses because the treatment occurred in end-stage patients with a prognosis of 4 to 6 weeks of life expectancy.
This article presents 3 cases illustrating the effects of IV-DCA treatment. All patients and/or guardians of patients gave consent for publication of their cases. These patients had a very poor prognosis and/or had not responded to conventional therapy. All patients derived meaningful benefits from the DCA therapy, with minimal side effects, no myelosuppression, and no organ toxicity. The patients in these case studies were treated in cooperation with the 3 authors who are naturopathic physicians. They developed individual protocols that included natural adjuvants, such as treatment with IV ascorbic acid/vitamin C (IVC) and natural neuroprotective agents.
CASE 1: COLON CANCER
A 79-year-old male sought therapy for metastatic colon cancer, with an original diagnosis of T3N1M0 sigmoid adenocarcinoma that was moderately differentiated. Comorbidities included a history of angina, myocardial infarction, quadruple coronary bypass, hypertension, hypercholesterolemia, and diverticulosis. He was initially treated with a left hemicolectomy, and colostomy, followed by 5-fluorouracil (5-FU) and irinotecan chemotherapy for approximately 6 months, and subsequent reversal of the colostomy.
The patient remained disease-free for approximately 3 years at which time the carcinoembryonic antigen (CEA) began to increase, and a computed tomography (CT) scan showed new liver and lung metastases. He was again treated with 5-FU and irinotecan chemotherapy for 3 cycles, which were ineffective and complicated by extreme fatigue and malaise. Chemotherapy was discontinued, and the patient was referred to palliative care.
The patient chose to undertake naturopathic treatment at one author’s clinic, beginning in December 2010. The therapy included homeopathic remedies and IVC at a dose of 75 g twice per week, with a subsequent increased energy level and weight gain. The medications and supplements at the time were pantoprazole, alfuzosin, metoprolol, ramipril, simvastatin, amlodipine, clopidogrel, vitamin D, vitamin B complex, acidophilus, and α-lipoic acid. The patient continued treatment through 2011 with good symptom control; however, his CEA continued to increase. A CT scan in November 2011 demonstrated extensive, diffuse, metastatic liver disease, and numerous enlarging pulmonary nodules consistent with interval disease progression. The patient then chose to undertake a trial course of IV DCA. Baseline blood tests were obtained, including CEA and liver enzymes, on December 19, 2011. The first dose of 3000 mg (41 mg/kg) of IV DCA was administered on December 22, 2011, together with 50 g of IVC. A CEA test was scheduled to be performed 4 weeks later but was mistakenly repeated 1 day after the infusion, together with the measurement of liver enzymes. A rapid reduction of liver enzymes and CEA were noted. (See Figure 1 and Figure 2.)
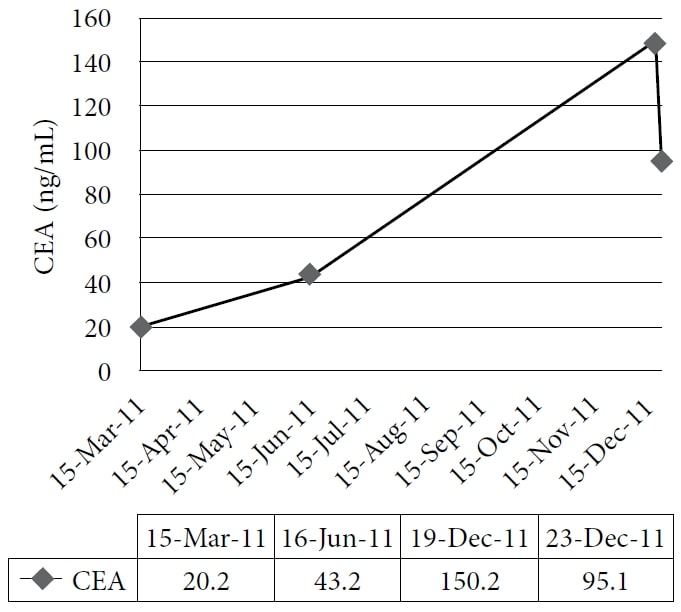
The figure shows the gradual increase of carcinoembryonic antigen (CEA) during naturopathic treatment and its sharp decrease after administration of intravenous dichloroacetate sodium (DCA).
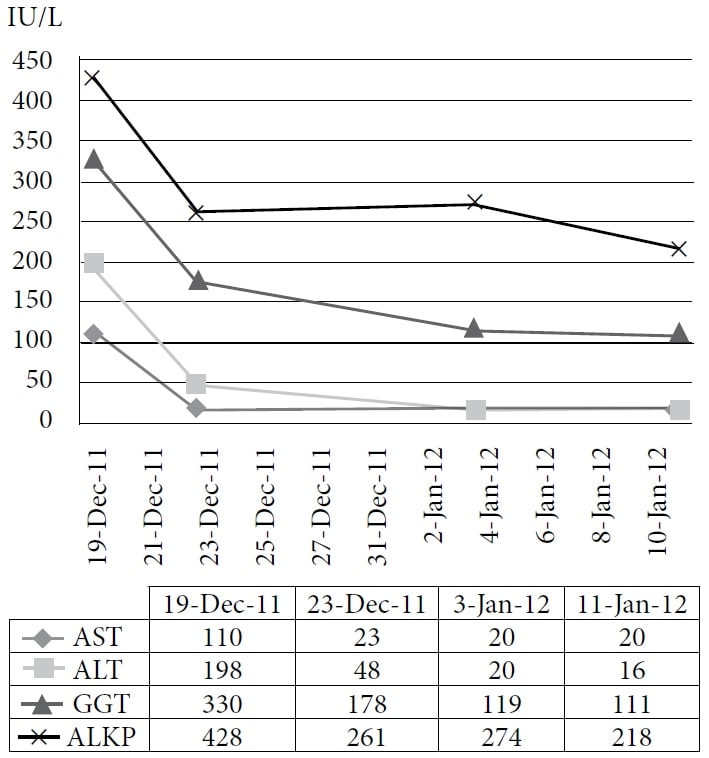
Abbreviations: AST = aspartate aminotransferase; ALT = alanine aminotransferase; GGT = γ-glutamyl transpeptidase; ALKP = alkaline phosphatase.
The figure shows the immediate sharp drop and the continued decline of liver enzymes with administration of IV DCA.
The second DCA infusion was given 6 days later at a dose of 3500 mg (48 mg/kg) together with 35 g of IVC. After the infusion, the patient noted new symptoms: chills, sweats, and fatigue. A third DCA infusion at a dose of 3700 mg (50 mg/kg) and 50 g of IVC were given 8 days after the second dose, with subsequent blood tests showing further improvements (Figure 2).
However, the patient again noted a brief period of fatigue and malaise after the infusion, and against his doctors’ advice, decided to stop treatment based on those side effects. A new abdominal ultrasound was performed 1 month after the DCA treatment. The report read, “Allowing for differences in imaging modalities, the appearance of the liver metastases has not significantly changed from the previous CT of the abdomen of November 10, 2011.” Lung metastases were not evaluated, because no respiratory symptoms were evident.
CASE 2: ANGIOSARCOMA
A 43-year-old male sought therapy for metastatic angiosarcoma of the right femur. The patient had a history of osteosarcoma of the right tibia, diagnosed at age 27 in 1995 and treated with resection and tumor-prosthesis reconstruction, followed by doxorubicin and cisplatin chemotherapy. This cancer was considered cured.
At the age of 39 in 2007, he consulted his doctor with pain and swelling above the right knee. Following magnetic resonance imaging (MRI) and a biopsy, a diagnosis of angiosarcoma was made. This cancer was believed to be a potential secondary malignancy caused by the prior chemotherapy. The patient received doxorubicin and ifosfamide chemotherapy, but the intended course was shortened because of infection and subsequent renal injury secondary to antibiotic treatment with vancomycin. He then underwent tumor resection and reconstruction in 2008.
In 2009, 1 year after that surgery, he developed lung metastases that were removed surgically with lung-wedge resection. Further lung metastases subsequently developed, for which the patient underwent 2 additional lung resections between 2009 and 2011. In August 2011, a left-iliac-wing metastasis was identified and was treated by excision and radiotherapy. In October 2011, a new pancreatic tail metastasis measuring 6.1 × 5.7 cm was diagnosed as the result of a CT scan and was treated immediately with radiotherapy. An enlarging, left-upperlobe, lung metastasis measuring 12 mm was also evident on CT scan.
In November 2011, the patient consulted one of the authors, a naturopathic doctor. The patient was treated with IV Viscum album (mistletoe extract) and IVC. Doses were escalated to 300 mg of viscum album and 75 g of IVC 3 times per week. He also received supplements, including vitamin D, modified citrus pectin, digestive enzymes, omega-3 fatty acids, bioperine, and artemisinin.
In January 2012, another CT scan revealed new, small spine metastases despite aggressive naturopathic therapy. The pancreatic tail mass had enlarged to 7.6 × 5.6 cm at 2 months postradiotherapy. Multiple new, small lung nodules were found. New lytic bone metastases were seen involving C3, T9, L2, and the manubrium.
Based on disease progression, the patient elected to begin IV DCA therapy in February 2012. Baseline blood-test results were significant for anemia (hemoglobin 101 g/L), mild renal failure (urea 12.6 mmol/L and creatinine 195 µmol/L), and mild γ-glutamyl transpeptidase (GGT) elevation (77 U/L). His alkaline phosphatase (ALKP) was normal. Prior to the start of the DCA, an MRI of the spine was performed as a baseline.
IV DCA was started at a dose of 3000 mg (47 mg/kg) weekly and escalated in a 2-week period to 5000 mg (47 mg/kg) twice per week. No side effects were observed. Concomitant to beginning the DCA, the patient also received stereotactic radiotherapy to the spine. After 2 months of IV DCA, in combination with the ongoing naturopathic treatments, a CT scan revealed stability in all lung metastases and shrinkage of the pancreatic metastasis from 7.6 × 5.6 cm to 5.9 × 5.2 cm. A subtle new area of lucency was seen at T12, with a mild increase in lucency of all other bone metastases. A technetium bone scan in April 2012 did not show corresponding activity in any of the areas of lucency seen on the CT. A new MRI of the spine confirmed a mixed response, with the greatest bone metastastic growth being a 1- to 2-mm increase.
Following a total of 4 months of IV DCA therapy, an MRI demonstrated stability for nearly all spine metastases, 2 of which were reported as being slightly larger, with no measurement specified. No new spine metastases were noted. A CT scan one month later, after 5 months of IV DCA, showed all lung nodules as being stable, and the pancreatic mass further diminished in size from 5.9 × 5.2 cm to 4.4 × 4.4 cm. No new intrathoracic or intra-abdominal metastases had occurred. Many of the existing bone metastases demonstrated a slight increase in lucency. ALKP remained normal, and no deterioration of cell counts or renal function had occurred. Although he continued the IV DCA therapy, the patient began a search for more aggressive treatment options, with the goal of achieving complete remission. Other than spinal stereotactic radiotherapy, no concurrent conventional therapy had been given at the same time as the IV DCA.
The patient remained clinically stable until September 2012, when he interrupted treatment with IV DCA after 8 months of therapy to travel to a private clinic in Germany for low-dose chemotherapy, with total-body hyperthermia and insulin potentiation.
CASE 3: NEUROENDOCRINE PANCREATIC CARCINOMA
A 10-year-old male with metastatic, neuroendocrine, pancreatic carcinoma was brought from Portugal by his parents to one author’s clinic in Canada for DCA treatment after failing conventional chemotherapy. He initially went to his doctor in Portugal with fatigue and anorexia. A diagnosis was made by ultrasound and biopsy, which revealed a pancreatic mass with abdominal-node metastases and extensive hepatic metastases occupying the entire liver parenchyma. Somatostatin receptor scintigraphy demonstrated a pancreatic lesion with high uptake but showed low uptake in all of the liver lesions. Based on a multidisciplinary case discussion with the pediatric oncology team at the regional cancer center in Lisbon, Portugal, 6 cycles of cisplatin and etoposide were selected as a primary treatment. A CT scan after 2 cycles of chemotherapy demonstrated a reduction in the metastases and primary tumor. By the fifth cycle, the oncologist noted a diminishing response based on new CT scans and blood markers, and the patient began to deteriorate clinically with weight loss, nausea, and vomiting. The possibility of highdose chemotherapy and liver transplant were ruled out by the transplant team. The therapy was abandoned after a total treatment of cisplatin at 600 mg/m2 and etoposide at 1800 mg/m2.
The patient arrived in Canada for assessment regarding DCA therapy 2 months later. His medications at the time included metoclopramide, ondansetron, fluconazole, loperamide, esomeprazole, naltrexone (4.5 mg) at bedtime, and paracetamol (acetaminophen). DCA at a dose of 2500 mg IV (74 mg/kg) and octreotide at a dose of 100 µg IV were initiated twice per week in the clinic setting. Octreotide was added with the hope of enhanced action against the main pancreatic tumor only; it had shown high uptake on a prior somatostatin receptor scan. Therapy was begun within 2 weeks of the most recent CT scan. Supportive IV treatment was provided concurrently and consisted of R-α-lipoic acid at 250 mg, amino acids, IVC, calcium, magnesium, vitamin B complex, and multitrace minerals, without copper. After 2 infusions, the IVC was escalated from 5 to 20 g after safety was confirmed by a normal serum-glucose-6-phosphate dehydrogenase (G6PD) level. Octreotide was increased to 100 µg by IV daily.
The patient experienced transient increased abdominal pain and new pain in his left shoulder tip 1 day after the infusions. He also reported an increase in his energy level. He continued to experience a temporary escalation of pain after each infusion, which was managed well with oral morphine, after discontinuing naltrexone. He was treated twice per week for 2 weeks in the office setting. New blood tests after 4 IV treatments revealed a reduction in alanine aminotransferase (ALT), stable aspartate aminotransferase (AST) and GGT, a large increase in lactic acid dehydrogenase (LDH), and a drop in hemoglobin (Table 1). He was then discharged to the care of his physicians in Portugal, who continued IV DCA therapy together with supportive IVC and IV α-lipoic acid, without complications.
| Name | Pre-DCA | After 4 Doses | After 12 Doses | Units |
| Hemoglobin | 91 | 71 | 81 | g/L |
| White blood cells | 5.7 | 8.2 | 8.2 | ×109/L |
| Platelets | 227 | 171 | 171 | ×109/L |
| Alkaline phosphatase | 188 | 206 | 122 | U/L |
| LDH | 988 | 1784 | 1449 | U/L |
| GGT | 169 | 171 | 76 | U/L |
| AST | 54 | 55 | 70 | U/L |
| ALT | 23 | 15 | 15 | U/L |
Abbreviations: LDH = lactic acid dehydrogenase; GGT = γ-glutamyl transpeptidase; AST = aspartate aminotransferase; ALT = alanine aminotransferase.
A CT scan after a total of 6 weeks of therapy showed shrinkage of the largest hepatic metastasis by 2 cm (Figures 3A and 3B). The second-largest metastasis also shrank by almost 2 cm (Figures 4A and 4B).
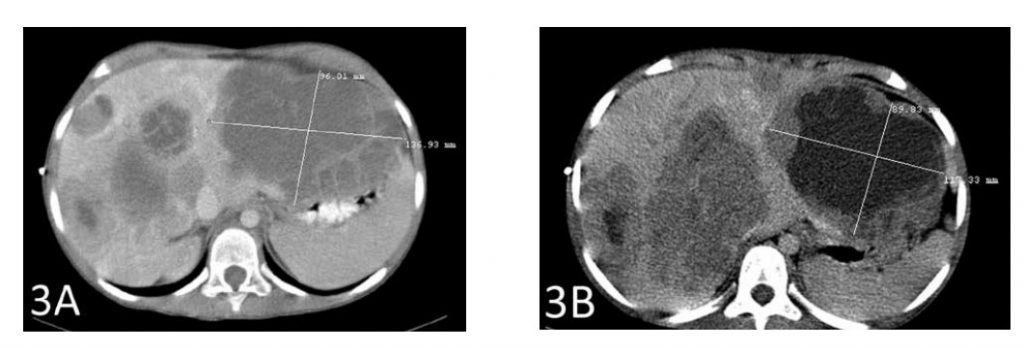
Figure 3A shows the largest metastasis prior to the start of therapy with IV DCA. Figure 3B shows the shrinkage of that tumor by 2 cm after therapy
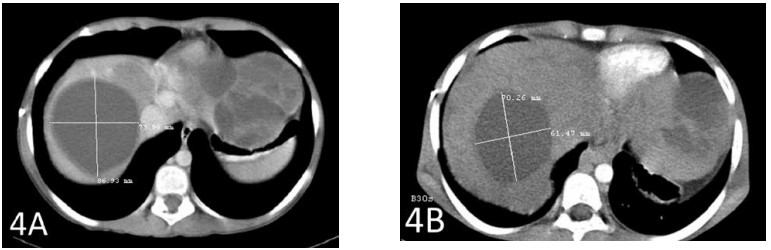
Figure 4A shows the second-largest metastasis prior to the start of therapy with IV DCA. Figure 4B shows the shrinkage of that tumor by almost 2 cm.
A new somatostatin scan (gallium 68 positron emission tomography) performed one week after the CT scan showed “no evidence of tumor lesions with increased expression of somatostatin receptors 2, 3, or 5.” Based on this finding, the IV octreotide was discontinued, and DCA therapy was maintained. The patient remained clinically stable with no changes in therapy for another 3 months. At that point, he requested to stop therapy because his personal goal was to achieve a functional status of ECOG level 0 (fully active with no restriction in physical abilities), and this goal was believed to be unattainable.
DISCUSSION
Three cases of IV DCA therapy for patients with advanced cancer have been presented in which the authors observed favorable clinical, biochemical, or radiologic responses.
Case 1 illustrates the biochemical response of metastatic colon cancer to IV DCA, combined with high-dose IVC. Previously, the patient’s CEA had climbed steadily while using IVC only for 10 months. Therefore, the decrease in CEA can be attributed to the DCA therapy. Because the literature reports potential for CEA reduction of more than 50% within 10 hours of a hepatic metastatectomy,40 a large drop in CEA at 1 day following any other therapy is plausible if the cancer has responded rapidly and should not be considered an erroneous result. Furthermore, the rapid decline of liver enzymes is consistent with reduced liver injury resulting from cancer-cell death or growth inhibition.
The post-DCA–treatment ultrasound reported no change in tumor sizes; however, no baseline scan was available immediately prior to initiation of the DCA treatment, and the comparison CT scan was performed 2 months prior to the start of therapy. Thus, no conclusions can be drawn about whether a reduction in tumor size occurred. Within the limitations of the times of the scans and of a CT-to-ultrasound comparison, the authors at the very least observed tumor stability. No conclusions can be drawn about durability of response because the patient discontinued therapy very early. The authors concluded that IV DCA appears to have activity in humans against colon adenocarcinoma.
Case 2 illustrates a partial response of metastatic angiosarcoma to IV DCA in combination with natural therapies. Despite pre-existing anemia and chronic renal failure, effective treatment was possible since DCA is not myelosuppressive or nephrotoxic. This patient experienced a reduction of a large pancreatic metastasis, which had not responded to prior radiotherapy. A reduction of more than 30% in size meets the definition of a partial response under the Response Evaluation Criteria in Solid Tumors (RECIST). Stability in the multiple lung metastases and nearly complete stability in existing bone metastases was the result. The response of the bone metastases could be attributable to concurrent stereotactic spinal radiotherapy, but the response of the other metastases cannot. Tiny, new bone metastases appeared during the initial 5-month course of DCA therapy. No new intrathoracic or intra-abdominal metastases appeared during DCA therapy, whereas lung and pancreatic metastases had grown prior to that therapy. The patient experienced no significant treatment-related side effects.
Mixed responses can be expected because of tumor heterogeneity. Another possible explanation of the enhanced response of the pancreatic metastasis compared with the lung metastases is that the DCA may have greater efficacy in preradiated tumors. This result has been reported previously.41 A partial response is encouraging given the poor prognosis of the patient, particularly because the treatment was welltolerated with no hematological, renal, or neurological toxicity. With gentler, nontoxic therapies, a partial response may be completely acceptable and need not be abandoned. The authors concluded that IV DCA appears to have activity in humans against angiosarcoma.
Case 3 illustrates tumor reduction with IV DCA when combined with high-dose IVC. This result is classified as stable disease according to the RECIST definition because tumor reduction was lower than 30%. Minimal side effects were observed. Based on the pre- and posttreatment octreotide scans, the shrinkage of large hepatic metastases cannot be attributed to the octreotide therapy; neither scan showed octreotide receptors in the hepatic metastases. In addition, the disappearance of octreotide receptors from the pancreatic tumor further emphasizes the lack of benefit related to octreotide therapy. Thus, the response can be attributed to administration of DCA, combined with natural medicines. A temporary flare-up of tumor-related pain is sometimes noted with IV DCA and may be a sign of tumor response with resulting inflammation. A sudden increase in the patient’s serum LDH is consistent with this effect. Because of the short duration of the assessment, no conclusion can be drawn about the durability of response. The authors concluded that IV DCA appears to have activity in humans against neuroendocrine pancreatic carcinoma.
The presented cases indicate that IV DCA is a promising cancer therapy. All patients derived meaningful benefits from their therapies, with minimal subjective side effects and no hematological, renal, or neurological toxicities despite the advanced stage of disease in all cases. Treatments such as high-dose IVC that were combined with DCA were supportive in terms of quality of life and may have had a synergistic effect with the IV DCA. However, it was clear that those treatments alone did not account for the tumor responses. It is interesting to note that all the patients in this case series used IVC with IV DCA. It is believed that IVC can exert an anticancer effect by immunomodulation42,43 or by induction of autophagy mediated through generation of H2O2 and ATP depletion.44-46 IVC has been shown to act as an adjuvant in other cancer therapies, such as gemcitabine in pancreatic carcinoma.47-49 Therefore, IVC may have a significant role as an adjuvant to IV DCA in the treatment of advanced cancers.
As of this writing, 4 active clinical trials are investigating the role of oral DCA as a cancer therapy, but no trials are currently investigating the route of IV administration. Because of the nonproprietary status of DCA, it is difficult for researchers to raise funds on the scale needed to conduct human trials. It is hoped that these cases illustrating the benefits of IV DCA will encourage more formal clinical investigations. Further in vitro and in vivo research is needed to help define the mechanisms of action of DCA and the specifics of tumor type and microenvironment that respond favorably to this drug. Clinical trials with patients who have tumors already known to respond favorably to DCA are warranted to assess the benefits of the drug better and to optimize the treatment regimen. DCA has shown promise in combination with some conventional chemotherapeutic agents, a benefit that also should be explored further.
CONCLUSION
Based on the literature and the authors’ clinical experience, off-label IV DCA is a promising treatment option for patients who fully understand and accept its risks and benefits, particularly those who have no conventional treatment options available. These case studies show that DCA has the potential to extend life without reducing patients’ quality of life with debilitating side effects or compromising physiological function, even for disease in a very advanced stage. In the cases presented, IV DCA therapy was of limited duration, making it difficult to estimate the degree of survival benefit. However, based on the authors’ experience of chronic use of oral DCA, with the longest current survivor being a 48-year-old male with glioblastoma who has been stable for 6 years on oral DCA with no conventional therapy, the authors believe that the drug offers potential for long-term stabilization and/or regression as well as substantial enhancement of survival. Given its affordability and low toxicity, DCA deserves further investigation.
ACKNOWLEDGEMENTS
The authors wish to thank Dr Humaira Khan for her assistance and also to thank the patients for their support and consent to publish their cases.
AUTHOR DISCLOSURE STATEMENT
Within their respective clinics, the authors administered individualized, integrative, adjunctive protocols to patients with cancer for a fee, including a cost for 1 or more of the medications listed in this publication. The clinics are owned by the authors and/or their family members. The authors received no other grants or financial support for this study.
REFERENCES
1 1. “Dichloroacetate cancer” search results. ClinicalTrials.gov Web site. http:// clinicaltrials.gov/ct2/results?term=dichloroacetate+cancer. Accessed July 22, 2014.
2 Stacpoole PW, Harman EM, Curry SH, Baumgartner TG, Misbin RI. Treatment of lactic acidosis with dichloroacetate. N Engl J Med. 1983;309(7):390-396.
3 Stacpoole PW, Lorenz AC, Thomas RG, Harman EM. Dichloroacetate in the treatment of lactic acidosis. Ann Intern Med. 1988;108(1):58-63.
4Stacpoole PW, Wright EC, Baumgartner TG, et al. A controlled clinical trial of dichloroacetate for treatment of lactic acidosis in adults: the DichloroacetateLactic Acidosis Study Group. N Engl J Med. 1992;327(22):1564-1569.
5 Stacpoole PW, Kurtz TL, Han Z, Langaee T. Role of dichloroacetate in the treatment of genetic mitochondrial diseases. Adv Drug Deliv Rev. 2008;60(13- 14):1478-1487.
6 Stacpoole PW, Kerr DS, Barnes C, et al. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117(5):1519-1531.
7 Kaufmann P, Engelstad K, Wei Y, et al. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66(3):324-330.
8 Brandsma D, Dorlo TP, Haanen JH, Beijnen JH, Boogerd W. Severe encephalopathy and polyneuropathy induced by dichloroacetate. J Neurol. 2010;257(12):2099-2100.
9 Stacpoole PW, Gilbert LR, Neiberger RE, et al. Evaluation of long-term treatment of children with congenital lactic acidosis with dichloroacetate. Pediatrics. 2008;121(5):e1223-e1228.
10 Bonnet S, Archer SL, Allalunis-Turner J, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11(1):37-51.
11 Madhok BM, Yeluri S, Perry SL, Hughes TA, Jayne DG. Dichloroacetate induces apoptosis and cell-cycle arrest in colorectal cancer cells. Br J Cancer. 2010;102(12):1746-1752.
12 Cao W, Yacoub S, Shiverick KT, et al. Dichloroacetate (DCA) sensitizes both wild-type and over expressing Bcl-2 prostate cancer cells in vitro to radiation. Prostate. 2008;68(11):1223-1231.
13 Saed GM, Fletcher NM, Jiang ZL, Abu-Soud HM, Diamond MP. Dichloroacetate induces apoptosis of epithelial ovarian cancer cells through a mechanism involving modulation of oxidative stress. Reprod Sci. 2011;18(12):1253-1261.
14 Vella S, Conti M, Tasso R, Cancedda R, Pagano A. Dichloroacetate inhibits neuroblastoma growth by specifically acting against malignant undifferentiated cells. Int J Cancer. 2012;130(7):1484-1493.
15 Fiebiger W, Olszewski U, Ulsperger E, Geissler K, Hamilton G. In vitro cytotoxicity of novel platinum-based drugs and dichloroacetate against lung carcinoid cell lines. Clin Transl Oncol. 2011;13(1):43-49.
16 Liu D, Liu S, Jing X, Li X, Li W, Huang Y. Necrosis of cervical carcinoma by dichloroacetate released from electrospun polylactide mats. Biomaterials. 2012;33(17):4362-4369.
17 Wong JY, Huggins GS, Debidda M, Munshi NC, De Vivo I. Dichloroacetate induces apoptosis in endometrial cancer cells. Gynecol Oncol. 2008;109(3):394- 402.
18Ishiguro T, Ishiguro R, Ishiguro M, Iwai S. Co-treatment of dichloroacetate, omeprazole and tamoxifen exhibited synergistically antiproliferative effect on malignant tumors: in vivo experiments and a case report. Hepatogastroenterology. 2012;59(116):994-996.
19 Sorokina LV, Pyatchanina TV, Didenko GV, Kaplia AA, Khyzhnyak SV. The influence of sodium dichloroacetate on the oxidative processes in sarcoma 37. Exp Oncol. 2011;33(4):216-221.
20 Kumar A, Kant S, Singh SM. Novel molecular mechanisms of antitumor action of dichloroacetate against T cell lymphoma: implication of altered glucose metabolism, pH homeostasis and cell survival regulation. Chem Biol Interact. 2012;199(1):29-37.
21 Sutendra G, Dromparis P, Kinnaird A, et al. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene. 2013;32(13):1638-1650.
22 Shahrzad S, Lacombe K, Adamcic U, Minhas K, Coomber BL. Sodium dichloroacetate (DCA) reduces apoptosis in colorectal tumor hypoxia. Cancer Lett. 2010;297(1):75-83.
23 Anderson KM, Jajeh J, Guinan P, Rubenstein M. In vitro effects of dichloroacetate and CO2 on hypoxic HeLa cells. Anticancer Res. 2009;29(11):4579-4588.
24. Olszewski U, Poulsen TT, Ulsperger E, Poulsen HS, Geissler K, Hamilton G. In vitro cytotoxicity of combinations of dichloroacetate with anticancer platinum compounds. Clin Pharmacol. 2010;2:177-183.
25 Xie J, Wang BS, Yu DH, et al. Dichloroacetate shifts the metabolism from glycolysis to glucose oxidation and exhibits synergistic growth inhibition with cisplatin in HeLa cells. Int J Oncol. 2011;38(2):409-417.
26 Medicor Cancer Centre’s observational DCA treatment data. Medicor Cancer Centres Web site. http://medicorcancer.com/dca_therapy/dca-therapy-datajuly-2009/. Updated July 1, 2009. Accessed July 22, 2014.
27 De Grandis D. Acetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: a short review. CNS Drugs. 2007;21(suppl 1):39-43.
28 Maestri A, De Pasquale Ceratti A, Cundari S, Zanna C, Cortesi E, Crino L. A pilot study on the effect of acetyl-L-carnitine in paclitaxel- and cisplatin-induced peripheral neuropathy. Tumori. 2005;91(2):135-138.
29Evans JD, Jacobs TF, Evans EW. Role of acetyl-L-carnitine in the treatment of diabetic peripheral neuropathy. Ann Pharmacother. 2008;42(11):1686-1691.
30 Di Cesare Mannelli L, Ghelardini C, Toscano A, Pacini A, Bartolini A. The neuropathy-protective agent acetyl-L-carnitine activates protein kinase C-gamma and MAPKs in a rat model of neuropathic pain. Neuroscience. 2010;165(4):1345-1352.
31 Mijnhout GS, Kollen BJ, Alkhalaf A, Kleefstra N, Bilo HJ. Alpha lipoic acid for symptomatic peripheral neuropathy in patients with diabetes: a meta-analysis of randomized controlled trials. Int J Endocrinol. 2012;2012:456279.
32 Vallianou N, Evangelopoulos A, Koutalas P. Alpha-lipoic acid and diabetic neuropathy. Rev Diabet Stud. 2009;6(4):230-236.
33 Liu F, Zhang Y, Yang M, et al. Curative effect of alpha-lipoic acid on peripheral neuropathy in type 2 diabetes: a clinical study [in Chinese]. Zhonghua Yi Xue Za Zhi. 2007;87(38):2706-2709.
34 Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the antioxidant alpha-lipoic acid: a 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia. 1995;38(12):1425-1433.
35 Winkler G, Kempler P. Pathomechanism of diabetic neuropathy: background of the pathogenesis-oriented therapy [in Hungarian]. Orv Hetil. 2010;151(24):971- 981.
36 Ang CD, Alviar MJ, Dans AL, et al. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;(3):CD004573.
37 Winkler G, Pal B, Nagybeganyi E, Ory I, Porochnavec M, Kempler P. Effectiveness of different benfotiamine dosage regimens in the treatment of painful diabetic neuropathy. Arzneimittelforschung. 1999;49(3):220-224.
38 Savasi I, Evans MK, Heigenhauser GJ, Spriet LL. Skeletal muscle metabolism is unaffected by DCA infusion and hyperoxia after onset of intense aerobic exercise. Am J Physiol Endocrinol Metab. 2002;283(1):E108-E115.
39 Shangraw RE, Lohan-Mannion D, Hayes A, Moriarty RM, Fu R, Robinson ST. Dichloroacetate stabilizes the intraoperative acid-base balance during liver transplantation. Liver Transpl. 2008;14(7):989-998.
40. Lokich J, Ellenberg S, Gerson B, Knox WE, Zamcheck N. Plasma clearance of carcinoembryonic antigen following hepatic metastatectomy. J Clin Oncol. 1984;2(5):462-465.
41 Khan A. Use of oral dichloroacetate for palliation of leg pain arising from metastatic poorly differentiated carcinoma: a case report. J Palliat Med. 2011;14(8):973-977.
42 See D, Mason S, Roshan R. Increased tumor necrosis factor alpha (TNF-alpha) and natural killer cell (NK) function using an integrative approach in late stage cancers. Immunol Invest. 2002;31(2):137-153.
43 Mikirova N, Casciari J, Rogers A, Taylor P. Effect of high-dose intravenous vitamin C on inflammation in cancer patients. J Transl Med. September 2012;10:189.
44 Chen P, Yu J, Chalmers B, et al. Pharmacological ascorbate induces cytotoxicity in prostate cancer cells through ATP depletion and induction of autophagy. Anticancer Drugs. 2012;23(4):437-444.
45 Chen P, Stone J, Sullivan G, Drisko JA, Chen Q. Anti-cancer effect of pharmacologic ascorbate and its interaction with supplementary parenteral glutathione in preclinical cancer models. Free Radic Biol Med. 2011;51(3):681- 687.
46 Deubzer B, Mayer F, Kuci Z, et al. H(2)O(2)-mediated cytotoxicity of pharmacologic ascorbate concentrations to neuroblastoma cells: potential role of lactate and ferritin. Cell Physiol Biochem. 2010;25(6):767-774.
47 Welsh JL, Wagner BA, van’t Erve TJ, et al. Pharmacological ascorbate with gemcitabine for the control of metastatic and node-positive pancreatic cancer (PACMAN): results from a phase I clinical trial. Cancer Chemother Pharmacol. 2013;71(3):765-775.
48 Monti DA, Mitchell E, Bazzan AJ, et al. Phase I evaluation of intravenous ascorbic acid in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. PloS One. 2012;7(1):e29794.
49 Cullen JJ, Spitz DR, Buettner GR. Comment on “Pharmacologic ascorbate synergizes with gemcitabine in preclinical models of pancreatic cancer” ie, all we are saying is, give C a chance. Free Radic Biol Med. 2011;50(12):1726-1727.
Related content: